Introduction
Intimal Sarcoma of the heart (IS) is a malignant tumor derived
from the intima. It develops predominantly intraluminally within
the large vessels and the heart. It is the least reported primary
tumor of the heart [1]. The histological classification of the IS is
still under debate [2]. The initial diagnosis can be delayed due to
the its clinical and radiological resemblance to the acute or chronic pulmonary thromboembolism [3]. Depending on the location
it can also mimic symptomatic pulmonary artery stenosis, mitral
valve stenosis or aortic dissection [4,5]. The initial treatment
usually comprises of the aggressive surgical resection followed by
chemotherapy and radiotherapy [6]. The prognosis still remains
poor [3,7]. The most common metastatic sites were described for
IS and include liver, kidneys, adrenal glands lungs and bones [8,9].
Central nervous system metastatic sites included several supratentorial regions as well as cerebellum [10-16].
Case description
63 year-old female patient with confirmed diagnosis of the left
atrium IS was admitted with history of aphasia and right hemiparesis. Two years prior, she underwent left atrium sarcoma resection followed by implantation of the biological mitral valve. Subsequently, chemotherapy of doxorubicin and dacarbazine was initiated and carried out during 5 months following cardiac surgery.
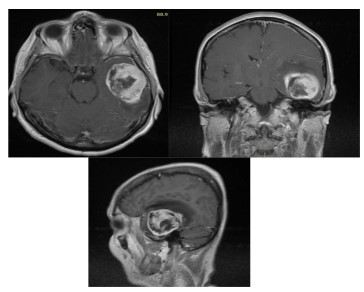
Brain MRI (Figure 1) revealed large middle fossa tumor. Left
temporal craniotomy was performed. As observed intraoperatively, the tumor was invading cortex of the temporal lobe. It presented with a distinct site of attachment to the floor of the middle
cranial fossa. Gross total removal of the tumor in one piece was
achieved and the skull base attachment site was extripated as
well as coagulated.
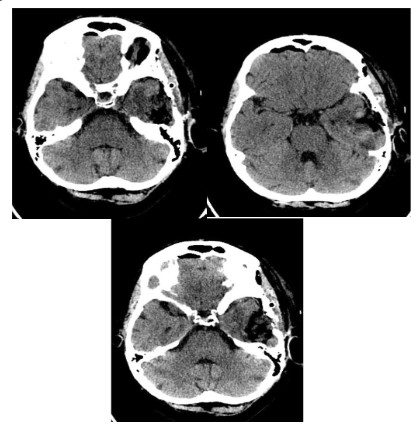
Postoperative period presented no complications. The control
brain CT was performed on 1st post-operative day (Figure 2). Patient was discharged on the 5th post-operative day in a stable
neurological condition.
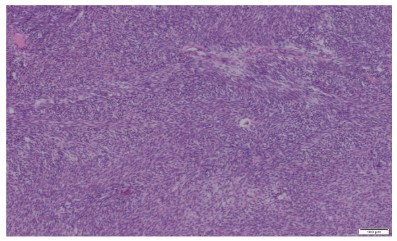
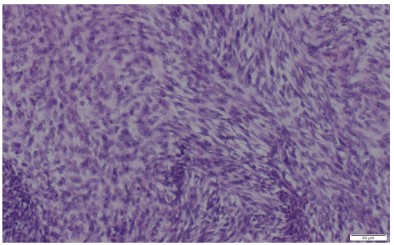
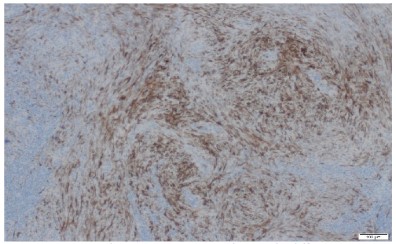
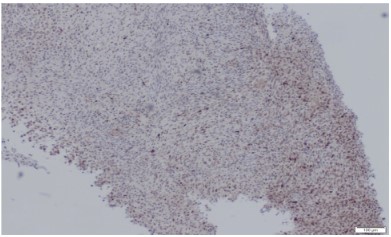
The pathologic diagnosis was established based on traditional pathological staining followed by immunochemistry. Mouse
Double Minute 2 Homolog (MDM2) staining was positive, CD34
negative and ERG were slightly positive. Fluorescence In Situ Hybridization (FISH) confirmed the presence of MDM2 amplification
which is very common in intimal sarcomas.
No gene fusion including NTRK1, NTRK2, NTRK3, ROS1, ALK
was detected. Additional mutations in NRAS, p.Gln61Leu (p.Q61L)
genes were detected. Based on the correlation with clinical data
and the initial diagnosis of the primary tumor, metastatic tumor
was confirmed to be high-grade intimal sarcoma.
The diagnosis was followed by radiotherapy planning. Follow-up MRI performed 3 moths post-operatively showed cerebral
mass of 20 x 7 mm diameter within left temporal lobe. The additional pathological mass of 22 x 16 mm diameter was found on
the chest MRI within the right inferior pulmonary artery (Figure
3). Additionally, ultrasound of the lower limb revealed 5cm large
tumor of the left leg. The general clinical status of the patient worsened. She died 6 months following surgery do to cardiopulmonary insufficiency.
Discussion
There are only a few reported cases handful of cases of cerebral
metastases of intimal sarcoma [10-16]. Metastases can cause the
embolisation of large brain vessels causing wide infarction regions
[14]. Multiple metastases were present supratentorially [11,15]
as well infratentorially [11]. Single IS metastasis at the time of presentation was observed within frontal lobe at parafalcine location
[12] or white matter of right periventricular area [16]. There were,
in literature, additional two cases of single metastasis at the time
of diagnosis with no specific cerebral location provided [13].
These lesions might be responsible for the onset of symptoms
[16] or might be found only during the autopsy [10,15]. The location of a single tumor with parallel MRI appearance including
gadolinum enhancement might initially suggest meningioma [12],
as in our case.
Here, the metastasis was located at floor of the middle cranial
fossa. Such location might be related to the metastatic invasion
of the carotid canal at the skull base. This could explain the site
of tumor attachment at the floor of the fossa, observed during
resection.
The approaches to the treatment of the brain metastases included total removal of a tumor [12,16] or radiation and/or palliative treatment [11,13].
Prognosis of these tumors still remains poor. The most optimal therapeutic approach includes aggressive surgical treatment,
followed by chemotherapy and radiotherapy. Radiotherapy might
include radiosurgery which is already used in treatment of some types of brain metastases [17]. The diagnosis of IS is extremely
challenging because there is no specific pattern of diagnostic markers [18]. Most common marker seems to be MM2 - but lacks specificity. Some attention has recently been drawn to more detailed
pathological profiling of these tumors, which might be helpful
while establishing new therapeutic targets. Especially mutations
in platelet-derived growth factor receptor alpha (PDGF-Ralpha)
gene, platelet-derived growth factor receptor beta (PDGF-Rbeta)
gene as well as epidermal growth factor receptor (EGF-R) [18,19].
Conclusion
We believe the SI, although rare, should be included in the differential diagnosis of the brain metastatic disease. The onset of
neurological symptoms in patients with diagnosed SI should raise
a suspicion of the brain involvement and provoke the broadening
of the diagnostics in order to continue treatment.
Conflict of interest disclosures: The authors have no financial
conflicts of interest to declare.
References
- Vinod P, Jabri A, Hegde V, Lahorra J, Cutler D. Functional Mitral
Stenosis: Imposture of Primary Cardiac Intimal Sarcoma. Cardiol
Res. 2018; 9: 307-313
- Kobayashi H, Kobayashi Y, Yuasa S, Okabe M, Yamada Y, et al. A
Case of Undifferentiated Sarcoma in the Superior Vena Cava and
Bilateral Cervical Veins. Am J Case Rep. 2018; 19: 1507-1514.
- Harbhajanka A, Dahoud W, Michael CW, Elliot R. Cytohistological
correlation, immunohistochemistry and Murine Double Minute
Clone 2 amplification of pulmonary artery intimal sarcoma: A case
report with review of literature. Diagn Cytopathol. 2018; 14.
- Yamamoto M, Hiroi M, Noguchi T, Orihashi K. Intramurally spreading aortic intimal sarcoma masquerading as ruptured aortic dissection. Interact Cardiovasc Thorac Surg. 2018; 26: 328-330.
- Manmadhan A, Malhotra SP, Weinberg CR, Reyentovich A, Latson
LA Jr, et al. Intimal spindle cell sarcoma masquerading as adult-onset symptomatic pulmonic stenosis: a case report and review of
the literature. J Cardiothorac Surg. 2017; 12: 93.
- Edquist M, Lui C, Kilimnik G, Karp H. Computed tomography imaging characteristics of primary atrial intimal sarcoma. Clin Imaging. 2018; 54: 112-115.
- García-Cabezas S, Centeno-Haro M, Espejo-Pérez S, CarmonaAsenjo E, Moreno-Vega AL, et al. Intimal sarcoma of the pulmonary artery with multiple lung metastases: Long-term survival case.
World J Clin Oncol. 2017; 8: 366-370.
- Sakata H, Suzuki K, Furuse H, Taniguchi H. Pulmonary Artery Intimal Sarcoma Invading the Left Lung. Intern Med. 2016; 55: 1397-1398.
- Osei-Agyemang T, Geks J, Wagner HJ, Feek U, Gerdes B. High-grade
intimal sarcoma in an aneurysm of the infrarenal aorta. Chirurg.
2004; 75: 823-827.
- Tanaka A, Shirasaka T, Okada K, Okita Y Aggressive multiple surgical interventions to pulmonary artery sarcoma. Eur J Cardiothorac
Surg. 2015; 47: 384-385.
- Scharl M, Bode B, Rushing E, Knuth A, Rordorf T. Uncommon case
of brain metastasis in a patient with a history of heavy smoking.
Curr Oncol. 2014; 21.
- Mecklai A, Rosenzweig B, Applebaum R, Axel L, Grossi E, Chan A,
Intimal sarcoma in the aortic arch partially obstructing the aorta
with metastasis to the brain. Saric M Tex Heart Inst J. 2014; 41:
433-436.
- Penel N, Taieb S, Ceugnart L, Dansin E, Hoguet D, et al. Report of
eight recent cases of locally advanced primary pulmonary artery
sarcomas: failure of Doxorubicin-based chemotherapy. J Thorac
Oncol. 2008; 3: 907-911.
- Sánchez-Muñoz, Hitt R, Artiles V, López A, Hernández R, et al.
Primary aortic sarcoma with widespread vascular embolic metastases. Eur J Intern Med. 2003; 14: 258-261.
- Araki Y, Tajima K, Yoshikawa M, Abe T, Suenaga Y. A case of primary
pulmonary intimal sarcoma of the pulmonary artery. Nihon Kyobu
Geka Gakkai Zasshi. 1997; 45: 1039-1043.
- Saith SE, Duzenli A, Zavaro D, Apergis G. Intimal (spindle cell) sarcoma of the left atrium presenting with abnormal neurological
examination. BMJ Case Rep. 2015; 2015.
- Soltys SG, Kalani MY, Cheshier SH, Szabo KA, Lo A, Chang SD. Stereotactic radiosurgery for a cardiac sarcoma: a case report. Technol Cancer Res Treat. 2008; 7: 363-368.
- Fu X, Niu W, Li J, Kiliti AJ, Al-Ahmadie HA, et al. Activating mutation
of PDGFRB gene in a rare cardiac undifferentiated intimal sarcoma of the left atrium: a case report. Oncotarget. 2017; 8: 81709-81716.
- Ito Y, Maeda D, Yoshida M, Yoshida A, Kudo-Asabe Y, et al. Cardiac
intimal sarcoma with PDGFRβ mutation and co-amplification of
PDGFRα and MDM2: an autopsy case analyzed by whole-exome
sequencing. Virchows Arch. 2017; 471: 423-428.