Introduction
Systemic Lupus Erythematosus (SLE) is a prototypic autoimmune disease characterized by the production of multiple pathogenic autoantibodies, in combination with diverse clinical manifestations [1]. Plenty of studies have indicated that apoptotic cells
with expression of altered or unaltered nuclear substance might
be a source of autoantigens in SLE [2-4]. In healthy individuals,
dying cells are cleared by macrophages at very early stages with
intact cell membrane, inducing anti-inflammatory cytokines, and
obviating any inflammation or immune response [5,6]. If they are
not promptly cleared, they may progress to late apoptotic cells
with disrupted membrane, releasing toxic and immunogenic intracellular contents, and fostering inflammation [7,8].
Esther Reefman et al found that IgG fractions from all of their
studied SLE patients could bind to late apoptotic cells and inhibit
their uptake by macrophages, indicating that the interference of
autoantibodies in the clearance of late apoptotic cells might be a
common denominator in the pathogenesis of SLE [9]. However, to
date the occurrence of late apoptosis has not been confirmed in
vivo, and something must have happened before large numbers of
unexpected late apoptotic cells emerged in the body. Early apoptotic cells are more likely to be the target of the autoimmunity in
SLE. Manfredi et al showed that affinity purified antiphospholipid
antibodies could recognize early apoptotic cells, facilitate apoptotic cell clearance by macrophages and trigger TNF-α release, indicating a pathogenic role in SLE [10]. Autoantibodies against early
apoptotic cells have also been detected in 62% anti-Ro60-positive
SLE patients, and the specificity of the Ro 60 epitope expressed
on apoptotic cells was determined by inhibition experiments with
recombinant and native Ro 60 [11]. In our earlier study, we detected autoantibodies against early apoptotic cells in active Lupus
Nephritis (LN) patients, whatever anti-SSA autoantibody positive
or negative, and found the prevalence rate in the whole study
group was 33.3%. Autoantibodies against early apoptotic cells
could be detected in anti-SSA negative LN patients, but patients
who were double positive for anti-SSA and anti-early apoptotic
cell antibody had significantly increased risk of poor short-term
outcome. Moreover, in our study, IgG presenting extensive binding capacity to early apoptotic cells were from 3 patients, all of
whom were anti-SSA antibody positive and antiphospholipid antibody negative [12]. To the best of our knowledge, the subsequent
consequence of the binding of IgG from patients with lupus to
auto antigens expressed on early apoptotic cells except phospholipid have not been investigated as yet.
During the process of fetal cardiocytes apoptosis, SSA/SSB antigens translocate to the cell surface, and mediate the phagocytosis
of the apoptotic fetal cardiocytes by nearby healthy cardiocytes.
In Neonatal Lupus Syndrome (NLS), maternal antibodies to SSA
and SSB transport across the placenta, bind to cognate antigen
expressed on apoptotic cardiocytes and decrease the clearance,
which may contribute to the development of autoimmune associated congenital heart block and fatal cardiomyopathy [13,14].
Since SSA might be the particular autoantigen exposed on early
apoptotic cells in non-neonatal SLE as elucidated hereinabove,
whether binding of autoantibodies to early apoptotic cells is one
of the mechanisms contributing to the pathologic cascade of non-neonatal SLE as they did in NLS is deserved to be studied.
In this study, we aimed to elucidate whether the binding of
IgG from LN patients with anti-SSA antibodies to early apoptotic
cells enhance complement activation, and affect phagocytosis by
macrophages.
Methods
IgG isolation
IgG from three LN patients which have been confirmed with
extensive binding to early apoptotic cells in our previous study
were used for functional assays [12]. Complete clinical data of
the three patients were collected upon presentation. They fulfilled the 1997 American College of Rheumatology revised criteria
for SLE [15]. The clinical characteristics of the three patients are
presented in Table 1. Informed consent was obtained for blood
sampling. The research was in compliance of the Declaration of
Helsinki. Ethical approval was obtained from hospital ethics committee of Shandong Provincial Hospital affiliated to Shandong University for this study.
Table 1: Clinical characteristics.
|
P5 |
P16 |
P23 |
| Gender |
M |
F |
M |
| Age (years) |
16 |
14 |
29 |
| Serum Creatinine (μmol/L) |
96 |
89 |
187 |
| Proteinuria (g/Day) |
3.23 |
5.64 |
10.17 |
| SLEDAI |
32 |
12 |
28 |
| ANA (titer) |
1:1000 |
1:1000 |
1:1000 |
| Anti-dsDNA (u/mL) |
734 |
210 |
297 |
| Anti-Sm |
+ |
+ |
+ |
| Anti-SSA |
+ |
+ |
+ |
| Anti-SSB |
+ |
- |
+ |
| Antiphosphollipid antibody |
- |
- |
- |
| % IgG binding to early apoptotic cells |
90.83 |
64.88 |
29.50 |
| Outcome after 1-year follow-up |
Death |
Remission |
Death |
aAbbreviation: SLEDAI: SLE Disease Activity Index.
IgG was purified from patient and control sera on protein G-Sepharose columns (Pharmacia) according to the manufacturer’s
recommendations. Briefly, IgG from serum was bound to protein
G columns, washed with 50-column volumes of Phosphate Buffered Saline (PBS), and eluted with 0.1 M glycine (pH 2.7). Eluted
IgG was neutralized by collection in 2M Tris-HCl (pH 9.0) and dialyzed against PBS.
Cell culture
The human T cell line Jurkat and promonocytic cell line THP-1
(Cell Resource Center, IBMS, CAMS/PUMC, China) were cultured
in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at
37°C in 5% CO2. THP-1 monocytes (2×105 cells/well) were stimulated with 100 nM phorbol 12-myristate 13-acetate (PMA; Sigma,
USA) for 48 h in 24-well plates to induce a macrophage phenotype.
Induction of apoptosis
Apoptosis was induced according to a previously described
method [12]. In brief, Jurkat cells were washed twice with serum free RPMI 1640, and then re suspended at a concentration of
1×106 cells/ml and irradiated with UVC light for 3 min. After UV irradiation, cells were cultured for 3 h in serum-free RPMI medium,
and stained with APC–labeled annexin V and 7-AAD (KeyGen
Biotech, Nanjing, China), which were then analyzed by flow cytometry. Since IgG from patients with lupus have been reported to
bind to late apoptotic cells extensively and inhibit phagocytosis,
we controlled the percentage of late apoptotic cells to a minim
level in this study. As described in our previous study, after UVC
irradiation and 3 hours of culture, about 50% of Jurkat cells were
early apoptotic, whereas less than 5% of Jurkat cells were late
apoptotic.
Complement activation assay
Normal human sera (NHS) used for complement binding
and activation were stored at -80°C in small aliquots. Patient or
control IgG was incubated with apoptotic cells for binding according to a previously described method [12]. In brief, 1×106 apoptotic cells were resuspended in 100 μl 3% BSA/PBS, and incubated
with 500 μg/ml of patient or control IgG at 4oC for 1 h. Washing
was repeated, and complement binding studies were performed
by incubation of 106 cells with 100 μl of medium containing 20%
human serum in TC buffer (140 mM NaCl, 2 mM CaCl2, 10 mM
Tris, pH 8.0 or 7.4, supplemented with 1 mM Mg2+ and 1% BSA) at
37°C for 30 min for C3c analyzing, 1h for C5b-9 analyzing, or 3h
for 7AAD staining. After incubation, apoptotic cells were washed
twice in TC buffer, stained with 1:100 dilutions of FITC-conjugated anti-human C3c (Abcam) at 4oC for 30 min, or incubated with
1:100 rabbit anti-human C5b-9 (Abcam) at 4oC for 30min and then
stained with 1:500 dilutions of FITC-conjugated goat anti-rabbit
secondary antibodies(Abcam) for flow cytometry analysis.
Phagocytosis assay
Prior to the induction of apoptosis, Jurkat cells were fluorescently labeled with carboxyfluorescein diacetate succinamidyl
ester (CFSE; Sigma, USA), according to a previously described
method with mild modification [16]. In brief, Jurkat cells were
washed three times and suspended in PBS at 1×107 cells/ml and
incubated for 30 minutes at 37°C with 5 μM CFSE. Cells were
washed and resuspended at 1×106 cells/ml in serum-free RPMI
culture medium, used for apoptosis induction, as described above.
THP-1 derived macrophages were washed gently with RPMI 1640
twice. For the phagocytosis assay, UVB-irradiated early apoptotic cells were incubated with 500 μg/ml of patient or control
IgG, and the cells were washed twice to remove nonbinding IgG.
Subsequently, apoptotic cells (5×105 cells/well) were incubated
with macrophages (2×105 cells/well) for 30 minutes at 37°C in
an atmosphere containing 5% CO2, in the presence or absence of
20% NHS. After co-incubation for 30 min, the cells were detached
from the surface with accutase (Invitrogen). Macrophages were
then stained with APC-conjugated monoclonal antibodies against
CD11b (BD Biosciences) and uptake was analyzed by 2-color flow
cytometry. CD11b-positive cells which represented the subset of
macrophages were under analysis, and the percentage of CD11b-positive cells that stained positive for CFSE was used as a measure for the percentage of macrophages which ingested apoptotic
cells.
Collection of supernatants and analysis of cytokines
Cytokines analysis induced by the phagocytosis of early apoptotic cells was preformed according to a previous described method
[6]. In brief, 5×105 early apoptotic cells were pre incubated with
patient IgG (4°C, 1h) and/or NHS (37°C, 30 min) as above, washed,
and then incubated with 2×105 adherent macrophages for 18 h at
in 300 μl fresh RPMI 1640 in 24-well plates [6]. Supernatants were
collected, and centrifuged at 2,000 rpm to remove particulate
debris, then were stored in aliquots at -80°C. Cytokine concentrations in the culture supernatants were determined by ELISA,
using the Quantikine immunoassays manufactured by R & D Systems. The cytokines analyzed were IL-1β and TNF-a. Assays were
performed according to the instructions provided with each kit.
Statistical analysis
Statistical software SPSS 22.0 (SPSS, Chicago, IL, USA) was employed for statistical analysis. Data were presented as means of
parallel measurements. Comparison between groups was performed using one way analysis of variance with Bonferroni’s correction for multiple comparisons as appropriate. Statistical significance was considered as p < 0.05.
Results
IgG enhances early complement activation on early apoptotic
cells
Complements have previously been reported to assemble on
the surface of early apoptotic cells. According to Gershov’s study,
evidence of C3 activation was detected on early apoptotic cells
by 30 min post incubation with NHS, relatively obvious Membrane Attack Complex (MAC) assembly was detected by 1h, and
MAC mediated cell lysis was observed over time [17]. Then in this
study, we analyzed C3c binding on early apoptotic cells at 30min
post incubation with NHS, 1h for C5b-9 binding, and 3h for 7AAD
staining.
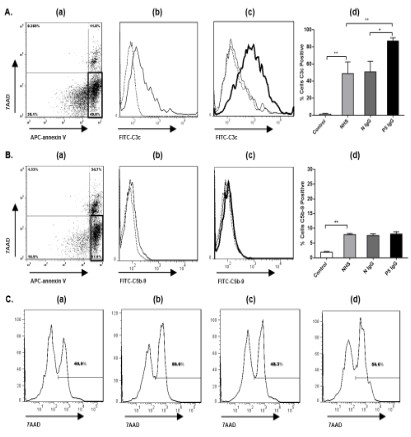
As shown in Figure 1A(b), 1B(b), incubating apoptotic cells with
20% NHS in TC buffer, 46.2% of early apoptotic cells were C3c positive at 30min, and 7.5% of early apoptotic cells were C5b-9 positive at 1h. As shown in Figure 1C(a-b), the percentage of 7AAD positive cells was higher when incubating apoptotic cells with NHS
compared with incubating apoptotic cells in TC buffer only. These
findings are consistent with Gershov’s results.
As shown in Figure 1A(c-d), 1B(c-d), 1C(c-d), when apoptotic
cells were pre incubated with IgG from patient P5, deposition of
C3c on the surface of early apoptotic cells was substantially amplified compared with IgG from healthy control (86.9 ± 4.0% vs.
50.7 ± 12.7%, p<0.05), but no more C5b-9 deposition was induced
by IgG from patient P5 compared with control IgG. Opsonization
of early apoptotic cells by IgG from patient P5 didn’t increase the
percentage of 7AAD positive cells in the presence of NHS compared with control IgG. These findings indicated that autoantibodies
from LN activate complement on early apoptotic cells, but auto-antibody-dependent complement activation are not efficiently to
induce cell lysis.
IgG from the other two patients P16 and P23 had no interference with complement activation on the surface of early apoptotic cells (data not shown).
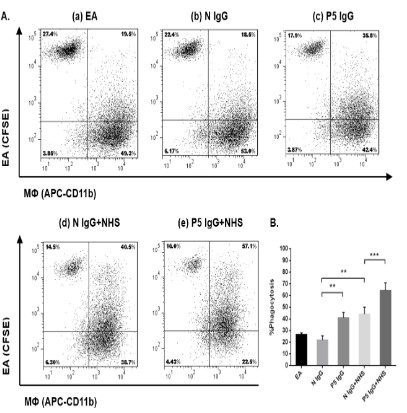
IgG enchances phagocytosis of early apoptotic cells
THP-1-derived macrophages were used to assess the uptake
of early apoptotic cells opsonized with IgG fractions from LN patients. As shown in Figure 2, in the absence of NHS, pre incubation of early apoptotic cells with control IgG fractions resulted in
a phagocytosis index comparable to that of cells pre incubated
with PBS alone (22.0 ± 3.6% vs. 26.5 ± 1.6%), opsonization of early
apoptotic cells with IgG fractions from patient P5 significantly enhanced the phagocytosis index to 41.0 ± 4.7%.
When the phagocytosis took place in the presence of 20% NHS,
uptake of early apoptotic cells was markedly increased, which is
consistent with the results of previous reports that complement
activation on early apoptotic cells facilitates phagocytosis. Compared with IgG control, opsonization of IgG from patient P5 significantly enhanced the phagocytosis in the presence of NHS (64.3
± 6.6% vs. 44.2 ± 6.1%, p<0.01), probably due to amplification of
complement activation on the surface of the apoptotic cells. Our
results demonstrated that IgG from LN patients could enhance
phagocytosis of early apoptotic cells directly or dependent on
complement activation.
IgG from the other two patients P16 and P23 had no interference with phagocytosis (data not shown).
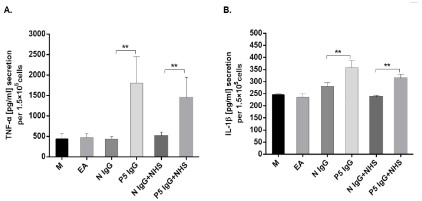
IgG induces secretion of proinflammatory cytokines by
macrophages
It has been shown that phagocytosis of apoptotic cells does not
stimulate the production of proinflammatory cytokines by macrophages. As shown in Figure 3, uptake of early apoptotic cells,
opsonized by NHS or not, induced limited amounts of TNF-a and
IL-1β secretion by macrophages. Opsonization of early apoptotic
cells by IgG from patient P5 stimulated TNF-a and IL-1β secretion
apparently compared with IgG control. When early apoptotic cells
coated with IgG from patient P5 were subsequently opsonized by
NHS, secretions of TNF-a and IL-1β were inhibited to some extent, which was probably due to anti-inflammatory property of
complement opsonized apoptotic cells. However, phagocytosis of
early apoptotic cells opsonized by IgG from patient P5 and NHS
still induced significantly higher TNF-a and IL-1β release compared with cells opsonized by control IgG and NHS.
IgG from the other two patients P16 and P23 had no interference with proinflammatory cytokines release (data not shown).
Discussion
Phospholipids and Ro 60 have been reported to be exposed
on the surface of early apoptotic cells and recognized by autoantibodies from SLE. Opsonization of early apoptotic cells by affinity
purified antiphospholipid antibodies enhanced recognition and
phagocytosis by macrophages, with massive TNF-α secretion. In
this study, we explored the subsequent effect of binding of IgG
from anti-SSA positive and antiphospholipid negative LN patients
on the fate of early apoptotic cells.
The interaction of complement proteins with apoptotic cells
has been studied in many papers. Apoptotic cells can bind C1q
[18,19], Mannose-Binding Lectin (MBL) [20], surfactant proteins
A and D [21] and C-reactive protein [17]. These complement proteins are opsonins marking apoptotic cells for uptake by phagocytes. Following the binding of the recognition molecules C1q and
MBL to their specific target, complement cascade can be slightly
activated, resulting in complement cleavage products deposition,
which are also efficient opsonins for phagocytosis [22]. In normal
circumstance, opsonized apoptotic cells are cleared quickly by
macrophages at the early stage, and they have little chance to
undergo complement-mediated cell lysis to induce inflammation.
When the phagocytosis was delayed, early apoptotic cells progressed, then complement activated extensively on the surface
of the apoptotic cells. At the meantime, cells acquired fluid phase
complement inhibitors to protect against excessive complement
activation and lysis [23]. Immune complexes are the prominent
initiators of complement classical pathway. Attali et al showed
the binding of rabbit anti-Jurkat antibodies to early apoptotic Jurkat cells enhanced C3 and C9 deposition, which indicated early
apoptotic cells were sensitive to antibody-dependent complement-mediated lysis [24]. To the best of our knowledge, this is
the first study to explore the interference of the binding of IgG
from lupus with complement activation on early apoptotic cells.
We observed limited deposition of C3b on early apoptotic cells
when incubated with NHS, and to a much lesser extent, C5b-9
on cells after a lag phase, which was consistent with the general
findings of previous studies. The binding of patient IgG substantially enhanced and sustained deposition of C3c on early apoptotic
cells, but unlike rabbit anti-Jurkat antibodies, patient IgG had no
obvious interference on the formation of MAC and complement-mediated cell lysis, which was probably due to the recruitment of
fluid phase complement inhibitors on apoptotic cells surface. The
reason for the difference between rabbit anti-Jurkat antibodies
and patient IgG might be attributed to variations in complement
activation capacity.
In the case of self antigens exposed on apoptotic cells, recognition by autoantibodies may have different and even conflicting interference with the phagocytosis by macrophages. Opsonization
of autoantibodies from SLE patients to late apoptotic cells inhibits
their uptake by macrophages via an inhibitory FcγRIIb-dependent
mechanism [9]. SLE IgG might decrease the uptake by blocking
antigen molecules on the surface of apoptotic cells that are necessary for recognition and facilitation of the phagocytosis, such
as C1q [25]. In NLS, binding of maternal anti-SSA antibodies to
apoptotic cardiocytes results in increased uPAR expression, which
is a kind of “don’t eat me” signals, then impairs the efferocytosis
[14]. Previous studies have demonstrated serum complement facilitates the uptake of apoptotic cells by phagocytes [22], which
was also observed in our study. In our study, patient IgG could
substantially enhance the clearance of early apoptotic cells in the
presence of NHS, which was probably due to amplified deposition
of complement components. Furthermore, patient IgG facilitated
the phagocytosis of early apoptotic cells independent of NHS.
Whether IgG promoted the phagocytosis through FcγR or regulation of molecule expression during early apoptosis process needs
to be investigated in future studies. If SSA was indeed the major
target antigen of our patient IgG exposed on early apoptotic cells,
our results suggested different roles of anti-SSA antibody played
in NLS and non-neonatal lupus.
Unlike the clearance of external pathogens, phagocytosis of
early apoptotic cells is a silent process inducing no secretion of
pro inflammatory cytokines to avoid inflammation. In our study,
we found few TNF-α and IL-1β secretion in the supernatants after
the phagocytosis of either early apoptotic cells or complement-opsonized early apoptotic cells. However, phagocytosis of patient
IgG opsonized early apoptotic cells induced massive TNF-α and
IL-1β secretion, even in the presence of NHS. Our results indicated
that opsonization of IgG from lupus promoted rapid clearance of
early apoptotic cells, but it changed early apoptotic cells from anti-inflammatory to pro inflammatory properties, which indicated
a contributor in the pathogenesis of lupus. Sophia et al demonstrated that anti-C1q from lupus induced a pro inflammatory phenotype in macrophages reversing the effects of C1q alone [26]. In
a recent research, the Ro 60 autoantigen could bind endogenous
retro elements and regulate inflammatory gene expression [27],
which might provide a direction to investigate the underling mechanisms of pro inflammatory property of patient IgG.
Among the three patients involved in our study, only IgG from
one patient (P5) exhibited functional effects, IgG from the other
two patients (P16 and P23) had no interference with either complement activation or phagocytosis. Considering IgG from P5
having the highest binding capacity to early apoptotic cells, we
thought the amount of binding IgG on the surface of apoptotic
cells might determine the subsequent functional effects. Then we
incubated apoptotic cells with higher concentrations of IgG from
P16 and P23 and performed the functional assays. We found that
in the higher concentration, more IgG bound to early apoptotic
cells, but still with no interference with either complement activation or phagocytosis (data not shown). One probable explanation
for the variation was that IgG from the three patients might have
different isotype profiles with difference in their ability to activate complement. Lupus is a highly heterogeneous and complex
disease, in our previous study exploring the functional role of anti
modified C-reactive protein autoantibodies, we also observed this
discrepancy [16]. Further work will be necessary to verify the prevalence rate of IgG with functional effects on early apoptosis in
lupus, and whether the binding of IgG to early apoptotic cells has
other functional consequences besides interference with complement activation and phagocytosis.
Limitations
Our study has a limitation in the small number of patients with
lupus nephritis, more patients are needed to validate our results
in future studies. Another limitation of our study is that although
we used IgG from anti-SSA positive and antiphospholipid negative
LN patients for the assays, the exact target antigen exposed on
early apoptotic cells is unknown, which needs to be confirmed in
further studies.
Conclusion
In conclusion, although IgG from a subset of anti-SSA positive LN patients enhanced early complement activation without
aggravating complement-mediated cell lysis on early apoptotic
cells and facilitated rapid phagocytosis, it seemed to change early
apoptotic cells from self to non self and fuel inflammation from
engulfing macrophages. This effect might exacerbate underlying
pathogenic mechanisms in lupus nephritis.
Declarations
Competing interest: The authors declare that they have no
known competing financial interests or personal relationships
that could have appeared to influence the work reported in this
paper.
Acknowledgments: Not applicable.
Funding sources: This study was supported by grants of National Natural Science Foundation of China (No. 81300587).
References
- D’Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007; 369: 587-96.
- Tax WJ, Kramers C, van Bruggen MC, Berden JH. Apoptosis, nucleosomes, and nephritis in systemic lupus erythematosus. Kidney Int.
1995; 48: 666-673.
- Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, et al.
Autoantigens are translocated into small apoptotic bodies during
early stages of apoptosis. Cell Death Differ. 2008; 15:183-191.
- Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in
systemic lupus erythematosus are clustered in two populations of
surface structures on apoptotic keratinocytes. J Exp Med. 1994;
179:1317-1330
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010; 140: 619-630.
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, et al.
Macrophages that have ingested apoptotic cells in vitro inhibitproinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest.
1998; 101: 890-898.
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFGE8-deficient mice. Science. 2004; 304: 1147-1150.
- Licht R, Dieker JW, Jacobs CW, Tax WJ, Berden JH. Decreased phagocytosis of apoptotic cells in diseased SLE mice. J Autoimmun.
2004; 22: 139-145.
- Reefman E, Horst G, Nijk MT, Limburg PC, Kallenberg CG, et al. Opsonization of late apoptotic cells by systemic lupus erythematosus
auto antibodies inhibits their uptake via an Fcgamma receptor-dependent mechanism. Arthritis Rheum. 2007; 56: 3399-3411.
- Manfredi AA, Rovere P, Galati G, Heltai S, Bozzolo E, et al. Apoptotic cell clearance in systemic lupus erythematosus. I. Opsonization
by antiphospholipid antibodies. Arthritis Rheum. 1998; 41: 205-
214.
- Reed JH, Jackson MW, Gordon TP. A B cell apotope of Ro 60 in
systemic lupus erythematosus. Arthritis Rheum. 2008; 58: 1125-
1129.
- Aktar K, Liu X, Yang X. Combination of anti-early apoptotic cell autoantibodies and anti-SSA autoantibodies in lupus nephritis. Cell
Mol Biol (Noisy-le-grand). 2018; 64: 48-54.
- Miranda-Carus ME, Askanase AD, Clancy RM, Di Donato F, Chou
TM, et al. Anti-SSA/Ro and anti-SSB/La auto antibodies bind the
surface of apoptotic fetal cardiocytes and promote secretion of
TNF-alpha by macrophages. J Immunol. 2000; 165: 5345-5351.
- Briassouli P, Komissarova EV, Clancy RM, Buyon JP. Role of the
urokinase plasminogen activator receptor in mediating impaired
efferocytosis of anti-SSA/Ro-bound apoptotic cardiocytes: Implications in the pathogenesis of congenital heart block. Circ Res. 2010;
107: 374-387.
- Hochberg MC. Updating the American College of Rheumatology
revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997; 40: 1725.
- Yang XW, Tan Y, Yu F, Zhao MH. Interference of antimodified C-reactive protein auto antibodies from lupus nephritis in the biofunctions of modified C-reactive protein. Hum Immunol. 2012; 73: 156-
163.
- Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to
apoptotic cells, protects the cells from assembly of the terminal
complement components, and sustains an anti-inflammatory innate immune response: implications for systemic autoimmunity. J
Exp Med. 2000; 192: 1353-1364.
- Korb LC, Ahearn JM. C1q binds directly and specifically to surface
blebs of apoptotic human keratinocytes: complement deficiency
and systemic lupus erythematosus revisited. J Immunol. 1997;
158: 4525-4528.
- Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, et al. Direct
binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002; 32: 1726-1736.
- Ogden CA, DeCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, et al. C1q and mannose binding lectin engagement of cell
surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001; 194: 781-795.
- Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, et al.
Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common
collectin receptor complex. J Immunol. 2002; 169: 3978-3986.
- Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement dependent clearance of apoptotic cells by human macrophages. J
Exp Med. 1998; 188: 2313-2320.
- Trouw LA, Bengtsson AA, Gelderman KA, Dahlback B, Sturfelt G,
et al. C4b-binding protein and factor H compensate for the loss
of membrane-bound complement inhibitors to protect apoptotic
cells against excessive complement attack. J Biol Chem. 2007; 282:
28540-28548.
- Attali G, Gancz D, Fishelson Z. Increased sensitivity of early apoptotic cells to complement-mediated lysis. Eur J Immunol. 2004; 34:
3236-3245.
- Pang Y, Yang XW, Song Y, Yu F, Zhao MH. Anti-C1q auto antibodies
from active lupus nephritis patients could inhibit the clearance of
apoptotic cells and complement classical pathway activation mediated by C1q in vitro. Immunobiology. 2014; 219: 980-989.
- Thanei S, Trendelenburg M. Anti-C1q Auto antibodies from Systemic Lupus Erythematosus Patients Induce a Proinflammatory Phenotype in Macrophages. J Immunol. 2016; 196: 2063-2074.
- Hung T, Pratt GA, Sundararaman B, Townsend MJ, Chaivorapol C,
et al. The Ro60 autoantigen binds endogenous retroelements and
regulates inflammatory gene expression. Science. 2015; 350: 455-
459.