Introduction
Cancer diseases have become a global challenge, and the
number of newly diagnosed cancer patients is increasing every
year [1]. Due to the undefined pathogenesis of cancer diseases
and the complexity of the cancer microenvironment, there has
been no effective treatment for multiple cancer diseases, such
as colon cancer [2]. Up to date, colon cancer is mainly treated
by surgery, accompanied with radiotherapy and chemotherapy,
but the therapeutic effect is unsatisfactory, especially for patients
with advanced metastases [3]. However, with the advancement
of medical science, treatment methods such as immunotherapy,
targeted therapy, intervention, and radiofrequency continue to
emerge, providing new treatment avenues for cancer patients [4,
5]. As an innovative treatment method, tumor immunotherapy
has become a hot spot in the field of tumor treatment research.
The concept of «cancer immunotherapy» is to fight the growth
and development of tumors with enhancing the body’s own immune system [6]. So far, cancer immunotherapy is mainly divided
into four categories, adoptive cell therapy, non-specific immune
activators, cancer vaccines and immune checkpoint inhibitors
(ICBs) [7]. Among these therapeutic approaches, ICBs treatment
stands at the point, and that can equip tumor treatment with novel and powerful «weapons» playing a huge role in the treatment
of various tumors [8]. In general, tumor-infiltrating tumor-killing lymphocytes, mainly is anti-tumor T lymphocytes, up-regulate the
immune checkpoints, such as PD-1, cytotoxic T lymphocyte-asso-
ciated antigen-4 (CTLA-4) and lymphocyte activation gene-3 (Tim-3) expression, which mediate the inhibitory signal greatly extinguish the activation and effect exert of tumor-killing lymphocytes,
thus promotes the tumor immune invasive [9-11]. PD-1, as an
inhibitory receptor, was first discovered in T cells, and that serve
a role of inhibitory signaling to prevent T cell excessive activation
in physiological state. However, in cancer diseases, PD-1 expression on anti-tumor cytotoxic T cells significantly up-regulated,
and inter-act with PD-L1 expressed on tumor or tumor-infiltrating
myeloid cells, greatly hinder the activation of cytotoxic T cells and
their anti-tumor function [12-14].
Except the adaptive T cells, PD-1 is also found expressed on
innate lymphoid cells (ILCs), such as ILC2s. In physiological situation, PD-1 expressed on ILC2s delivers inhibitory signaling, which
prevent the inflammatory cytokine secretion, thus sustaining
the homeostasis of body [15,16]. ILC2s, a subset of ILCs, do not
express antigen-specific receptor, but express transcription factors RORα and GATA3, and secret type 2 cytokine IL-4, IL-5, IL-9
and IL-13. Previous studies have shown that ILC2s mainly participate in the anti-helminth response and the development of allergic diseases like asthma [17,18]. Recent years, the role of ILC2s
in tumor diseases has been discovered, and studies have proved
that ILC2s serve as a double-edged sword in the development of
tumor diseases [19,20]. Here, we found that tumor cells can up-regulate the expression of PD-1 on ILC2s, which greatly inhibits
the activation and function of ILC2s. Neutralizing antibody PD-1
significantly prevent the tumor growth and restore the ILC2s activation inhibitory, which demonstrates the potential anti-tumor
role of ILC2s, and that maybe provide a useful direction to ILC2s-based immunotherapy to cancer diseases.
Material and methods
Reagents
Mouse anti-CD45, anti-Lineage, anti-CD90.2, anti-ST2, anti-PD-1 flow cytometry antibodies were purchased from Biolegend (San Diego, CA, USA). IL-4, IL-5, IL-9, IL-13 primer synthesis
by Shenggong Biotech Co., Ltd (Shanghai, China). IL-4, IL-5, IL-9,
IL-13 ELISA kits and the FITC-AnnexinV Apoptosis Detection Kit
were bought from Multi Sciences (Lianke, China) Biotech Co., Ltd.
(Hangzhou, China). Mouse PD-1 neutralizing antibody was bought
from R&D systems (Minnesota, USA). Cell culture medium (1640
and DMEM) and fetal bovine serum (FBS) was obtained from
Gibco Life Technologies (New York, USA).
Cells and animals
6-8 weeks old female BALB/c mice and nude mice were purchased from Yangzhou University animal center (Yangzhou, China)
and Jiangsu university animal center (Zhenjiang, China). All mice
were housed in the Animal Center of Jiangsu University in compliance with the guidelines outlined in the Guide for the Care and
Use of Laboratory Animals. All methods and protocols were approved by the Animal Care and Use Committee of Jiangsu University. MC38 were cultured in DMEM medium supplemented
with 10% FBS and 10 U/mL penicillin, and 10 U/mL streptomycin
(Gibco, NY, USA). ILC2s were isolated from mouse spleen by magnetic bead sorting and flow cytometry sorting, and the purity was identified by flow cytometry (FCM) analysis. Isolated ILC2s
were cultured in RPMI-1640 medium supplemented with 10% FBS
and 10 U/mL penicillin, and 10 U/mL streptomycin at 37°C in a
humidified atmosphere containing 5% CO
2.
Construction of the MC38 tumor-bearing model
To building the MC38 transplantation mice tumor model, we
treated 5×105 MC38 tumor cells to mice by subcutaneous injection. Monitoring the tumor size from day 7 and anti-PD-1 were
treated to the MC38 tumor-bearing mice to regulate the tumor
growth by intravenous injection. The length (L), diameter, and
width (W) of the tumor were tested with a vernier caliper, and the
volume of the tumor calculated using formula V = π × L × W2/6.
Enzyme-linked Immunosorbent Assay (ELISA)
Cell culture supernatants were collected. IL-4, IL-5, IL-9 and IL-13 were measured by ELISA kits according to the manufacturer’s
instructions.
RNA extraction and real-time fluorescence quantitative PCR
(RT-qPCR)
Total RNA was extractedfrom ILC2s using TRIzol reagent according to the manufacturer’s protocol and transcribed into first-strand cDNA. After cDNA synthesis, real-time PCR was performed
with iQ SYBR Green Supermix (Bio-Rad, Shanghai, China) and a
Real-Time PCR System (Bio-Rad, Hong Kong). β-Actin was included
as an internal control, and gene expression was quantified relative to β-actin. The primer sequences are shown: 5′-GCTAT-TGATGGGTCTCACCC-3′ reverse: 5′-CAGGACGTCAAGGTA-CAGGA-3′; IL-5: forward: 5′-AGGATGCTTCTGCACTTGAG-3′ reverse: 5′-CCTCATCGTCTCATTGCTTG-3′; IL-9: forward: 5′-CACAAATGCACCTTCTGGGACA-3′ reverse: 5′-TCACTCCAACGATACGGTCCTT-3′; IL-13: forward: 5′-TGAGCAACATCACACAAGACC-3′ reverse: 5′-AGGCCATGCAATATCCTCTG-3′.
Apoptosis analysis
Collecting MC38 tumor cells in the co-culture system, MC38
cells were stained with AnnexinV/PI Apoptosis Detection Kit according to the manufacturer’s protocol. Briefly, MC38 cells were
resuspended in binding buffer. Then, 10ul of FITC-AnnexinV and
20ul of APC-PI were mixed with the cells and incubated for 20 min
at room temperature. Adding another 200ul wash buffer to resuspend the incubated cells for FCM detecting, and the data were
analysed using Flow Jo software.
Flow cytometry
Tumor tissues were digested by the solution containing 0.5 mg/
mL collagenase V, 0.2 mg/mL hyaluronidase, and 0.015 mg/mL
DNase I (Sigma, St. Louis, USA) for half an hour in cell incubator.
Then tumor tissue digestion solution were filtered through 200
um strainer to get the digested single cells. Getting 5×105 digested single cells to stain with fluorescence antibodies. Mouse FITC-CD45, BV421-LIneage, PE-CD90.2 and APC-ST2 were applied to
detect the percentage of ILC2s in the tumor tissue of tumor-bearing mice and human samples. PEcy7-PD-1 was used to analyze
the expression level of PD-1 on tumor-infiltrating ILC2s. All the
procedures according to the standard flow cytometry staining
instructions and surface-stained samples were kept at 4oC for 15
min. Then add 1 ml PBS to wash the samples, cells were analysed by FCM using a BD FACSCalibur. The data were analysed using
FlowJo software.
Statistical analysis
All statistical analyses were performed using GraphPad Prism
5 software. Data are expressed as the mean SD. Comparisons
between groups were performed using paired t-test or one-way
ANOVA with Bonferroni correction. values less than 0.05 were
considered statistically significant.
Results
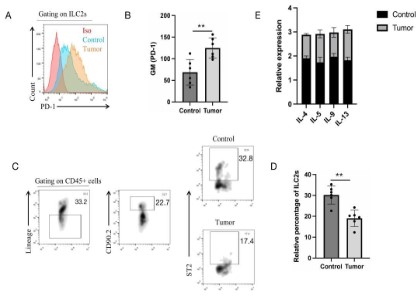
PD-1 is highly up-regulated in tumor-related ILC2s
Recent years, a great number of studies have demonstrated
that PD-1 serves as an important role in the regulation of immune
response especially in tumor diseases, which greatly hinder the
anti-tumor role of tumor-killing cells by delivering the inhibitory
signal [21]. Previous studies were all focused on the role of PD-1
on adaptive immune response T cells, but little known about the
PD-1 expression to ILCs, including ILC2s[22]. Here, we found that
the PD-1 expression in MC38 tumor-bearing mice tumor draining
lymph nodes (dLNs) ILC2s highly up-regulated (Figure 1 A and B).
Furthermore, the percentage of ILC2s in MC38-bearing mice dLNs
showed a low percentage compared to normal mice corresponding dLNs (Figure 1C and D). RT-PCR results were also showed that
ILC2s-related cytokines IL-4, IL-5, IL-9 and IL-13 in MC38-bearing
mice dLNs were greatly down-regulated compared to control mice
(Figure 1E). These results indicated that tumors have an effect on
the PD-1 expression on ILC2s, and that might inhibit the activation
and function exertion of ILC2s.
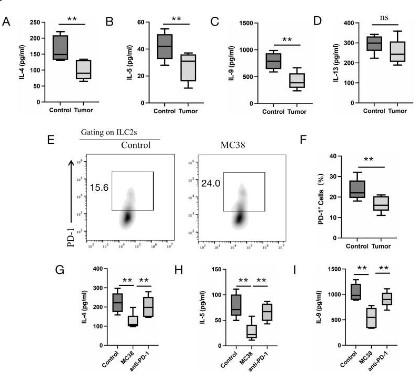
Tumor cells inhibit the function of ILC2s by up-regulating PD-1
expression in vitro
To investigate the function of ILC2s from MC38 tumor-bearing
mice, we isolated the ILC2s from the dLNs of MC38 tumor-bearing
mice and normal mice. Isolated ILC2s cultured in the 96 plates for 3
days, ELISA was used to detect the effect or cytokine secretion level of ILC2s. The results showed that ILC2s from MC38 tumor-bearing
mice dLNs greatly down-regulated the secretion ability of IL-4, IL-5, IL-9 compared to ILC2s from normal mice corresponding dLNs
(Figure 2A, B and C), but it has no significant difference of IL-13 secretion was found (Figure 2D). To further mimic the effect of tumor
microenvironment on ILC2s, we co-cultured isolated ILC2s with
MC38 cells for 24 h, flow cytometry was used to detect the
PD-1 expression on ILC2s. The result showed that PD-1 expression
greatly up-regulated on ILC2s when co-cultured with MC38 cells
(Figure 2E and F). Addition of anti-PD-1 could restore the function
of ILC2s by up-regulating the effect or cytokine secretion (Figure
2G, H and I). These results demonstrated that in tumor microenvironment, tumor cells could inhibit the function of ILC2s by increasing the expression of PD-1 on ILC2s.
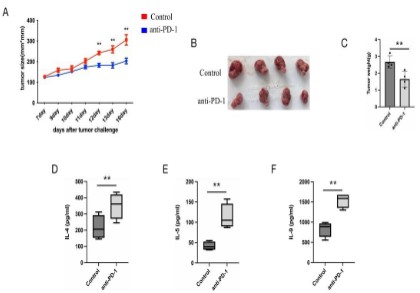
Administration of anti-PD-1 can restore the percentage and
function of ILC2s in vivo
To further investigate the up-regulation of PD-1 on ILC2s could
restrain the ILC2s function, we treated MC38 tumor-bearing nude
mice (lacking T and B cells) with anti-PD-1 antibody, at day 5 to
day 10 after tumor implanted. The results showed that the administration of anti-PD-1 greatly inhibit the growth of tumors of
MC38 tumor-bearing nude mice (Figure 3A, B and C). To detect if
anti-PD-1 reverse the ILC2s function inhibition effect, we isolated
the ILC2s from dLNs and cultured for 3 days. ELISA was used to
detect the ILC2s-related cytokines IL-4, IL-5, IL-9, result showed
that these cytokine were up-regulated in the anti-PD-1 treated
MC38 tumor-bearing nude mice dLNs ILC2s compared to control
mice (Figure 3D, E and F). These results preliminarily proved the
negative effect of tumor cells on tumor-infiltrating ILC2s by up-regulating PD-1 expression on them.
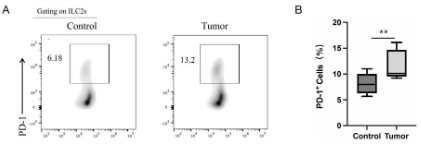
PD-1 is up-regulated in peripheral blood ILC2s of colon cancer
patient
To explore whether PD-1 was up-regulated on human colon
cancer patient, we detected the PD-1 expression on the peripheral blood ILC2s of colon cancer patient. Flow cytometry result
showed that PD-1 was greatly up-regulated on the peripheral
blood ILC2s of colon cancer patient compared to healthy donor
(Figure 4A and B), which is consistent with mice research results.
Our results preliminarily demonstrated that tumor can regulate
the PD-1 expression on ILC2s, and inhibited ILC2s function, thus
affect the tumor-regulating role of ILC2s.
Discussion & conclusion
In this study, we found that the PD-1 expression on tumor-infiltrating ILC2s greatly up-regulated, which push these ILC2s towards to an exhausted phenotype, such as the down-regulation
of effect or cytokine IL-4, IL-5, IL-9 and IL-13. Furthermore, in vitro
study showed that colon cancer cell line MC38 could up-regulate
the expression of PD-1 on ILC2s when co-cultured with isolated
ILC2s, and prevent the cytokine secretion from ILC2s, which could
reversed by adding anti-PD-1. In vivo study demonstrated that
the injection of neutralizing antibody PD-1 prevented the tumor
growth of MC38-bearing nude mice, and greatly release tumor-infiltrating ILC2s from inhibitory situation. Our studies firstly demonstrated that the regulation role of tumor cells on the PD-1
expression on tumor-infiltrating ILC2s, providing a new insight in
the ILC2s-based tumor immunotherapy.
PD-1, as an inhibitory molecular, mainly expresses on lymphoid
cells, and that interacts with PD-L1 or PD-L2 to deliver the inhibitory signal to prevent the excessive activation of immune cells
in physiology situation [23]. As an immune checkpoint, PD-1 prevents the immune cells excessive activation-induced autoimmune
diseases through two mechanism, that promotes the apoptosis
(programmed cell death) of antigen-specific T cells in lymph nodes
and reduces the apoptosis of immunosuppressive regulatory T
cells. It is indeed benefit in the physiology body. However, recent
studies have proved that PD-1 and their ligands are all up-regulated in multiple tumors, and that greatly inhibits the anti-tumor
function of tumor-inhibiting Cytotoxic T Lymphocytes (CTL) and
promotes the tumor growth [24-26]. Up to date, the study on PD-1-based immunotherapy has achieved great success, and some
PD-1 inhibitors showed powerful therapeutic effects on several
cancer diseases [27,28]. Although it is also necessary to further
explore the full mechanism on the PD-1 related tumor immune
inhibition. Here we found that tumor cells could also up-regulate
the PD-1 expression on innate lymphoid cells, ILC2s, that provide
a new insight in the interact of tumor cells with innate lymphoid
cells in tumor microenvironment.
ILC2s, a subset of innate lymphoid cells, were initially proved
to have an important role in the development of allergic diseases
and anti-helminth response [29]. Recent years, the role of ILC2s in
tumor diseases was achieved more and more attention. Studies
have proved that ILC2s have a dual role in tumors, so more studies need to be done to detect the real or exactly function in the
development of tumor diseases [30,31]. ILC2s are characterized
by secreting type 2 cytokines, and the role of ILC2s-derived IL-4,
IL-5, IL-9 and IL-13 have been reported. It is reported that IL-13
secreted by ILC2s can promote the activation of Myeloid-Derived
Suppressor Cells (MDSCs) and tumor-promoting M2 macrophage,
which greatly push the emergence of immunosuppressive tumor
microenvironment and promote the tumor growth [32]. Furthermore, ILC2s-derived IL-5 can recruit anti-tumor CD8 T cells to tumor core to exert tumor-killing effect by activating tumor-infiltrating eosinophils to secret T cell chemokines. Recent a publication
showed that ILC2s-derived IL-9 could inhibit the colorectal cancer
development by activating CD8 T cells [16,19]. These studies demonstrated that ILC2s-derived cytokines played an important role
in the regulation of tumor development. PD-1 expressed on ILC2s,
delivers inhibitory signal greatly prevent the activation and effect
or cytokine secretion of ILC2s. Here, we found that tumor cells
up-regulated PD-1 expression on ILC2s significantly inhibited the
activation of ILC2s and greatly decreased the cytokine secretion
from ILC2s, thus greatly hindering the regulating role of ILC2s on
tumors.
In conclusion, our results demonstrated that colon cancer cells
inhibited the activation of ILC2s by up-regulating the PD-1 expression on them, and inhibited the tumor development regulating
factors secretion, thus lose the anti-tumor function to colon cancer. Our results preliminary clarified the regulatory role of tumor
cells on ILC2s, providing a new approach on ILC2s-based immunotherapy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: All authors provided consent for publication.
Availability of supporting data: The conclusion figures have
been uploaded and the tables supporting the view of this review
have been listed in the article.
Conflict of interests: The authors declare that they have no
competing interests.
Funding: This work is supported by The scientific research development fund of Kangda College of Nanjing Medical University
(grant no. KD2021KYJJZD029).
Authors’ contributions: XX and HGZ wrote the manuscript.
BM, JC and JXD draw the figures and tables. WJZ designed the
study, drafted and revised the manuscript. All authors read and
approved the final manuscript.
References
- Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence
and Mortality Rates and Trends-An Update. Cancer Epidemiol Biomarkers Prev. 2016; 25: 16-27.
- Mun EJ, Babiker HM, Weinberg U, Kirson ED, Von Hoff DD, et al. Tumor-Treating Fields: A Fourth Modality in Cancer Treatment. Clin
Cancer Res. 2018; 24: 266-275.
- Labianca R, Beretta GD, Kildani B, Milesi L, Merlin F, Mosconi S, et
al. Colon cancer. Crit Rev Oncol Hematol. 2010; 74: 106-133.
- Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer
with specificity. Eur J Pharmacol. 2018; 834: 188-196.
- Tsimberidou, AM, Fountzilas E, Nikanjam M, Kurzrock R, et al. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat Rev. 2020; 86: 102019.
- Riley RS, June CH, Langer R, Mitchell MJ, et al. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019; 18:
175-196.
- Kennedy LB, Salama AKS. A review of cancer immune therapy toxicity. CA Cancer J Clin. 2020; 70: 86-104.
- López-Soto A, Gonzalez S, Folgueras AR. IFN Signaling and ICB Resistance: Time is on Tumor’s Side. Trends Cancer. 2017; 3: 161-163.
- Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target
in immunotherapy. Blood. 2018; 131: 58-67
- Han Y, Liu D, Li L. PD-1/PD-L1pathway: current researches in cancer. Am J Cancer Res. 2020; 10: 727-742.
- Zhao L, Cheng S, Fan L, Zhang B, Xu S et al. TIM-3: An update on
immunotherapy. Int Immuno pharmacol. 2021; 99: 107933.
- Larsen TV, Hussmann D, Nielsen AL. PD-L1and PD-L2 expression
correlated genes in non-small-cell lung cancer. Cancer Commun
(Lond). 2019; 39: 30.
- Wu Y. PD-L1Distribution and Perspective for Cancer Immunotherapy-Blockade, Knock down, or Inhibition. Front Immunol. 2019;
10: 2022.
- Wang X, Teng F, Kong L, Yu J, et al. PD-L1expression in human cancers and its association with clinical outcomes. Onco Targets Ther.
2016; 9: 5023-5039.
- Helou DG, Shafiei-Jahani P, Lo R, Howard E, Hurrell BP, Galle-Treger
L et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist Treatment ameliorates airway hyper reactivity. Nat Commun.
2020; 11: 3998.
- Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, et al. ILC2 simplify PD-1
blockade by activating tissue-specific cancer immunity. Nature.
2020; 579: 130-135.
- Bartemes KR, Kita H. Roles of innate lymphoid cells (ILCs) in allergic
diseases: The10-year anniversary for ILC2s. J Allergy Clin Immunol.
202; 147: 1531-1547.
- Bouchery T, Le Gros G, Harris N. ILC2s-Trail blazers in the Host Response Against Intestinal Helminths. Front Immunol. 2019; 10: 623.
- Wan J, Wu Y, Huang L, Tian Y, Ji X, et al. ILC2-derived IL-9 inhibits
colorectal cancer progression by activating CD8(+)T cells. Cancer
Lett. 2021; 502: 34-43.
- Trabanelli S, Chevalier MF, Derré L, Jandus C, et al. The pro-and
anti-tumor role of ILC2s. Semin Immunol. 2019; 41: 101276.
- Thommen DS, Schumacher TN. T Cell Dysfunction in Cancer. Cancer Cell. 2018; 33: 547-562.
- Taylor S, Huang Y, Mallett G, Stathopoulou C, Felizardo TC, et al.
PD-1 regulates KLRG1 (+) group 2 innate lymphoid cells. J Exp Med.
2017; 214: 1663-1678.
- Kamada T, Togashi Y, Tay C, Nishikawa H. PD-1(+) regulatory T cell
samplified by PD-1blockade promote hyper progression of cancer.
Proc Natl Acad Sci U S A. 2019; 116: 9999-10008.
- Ai L, Xu A, Xu J. Roles of PD-1/PD-L1Pathway: Signaling, Cancer,
and Beyond. Adv Exp Med Biol. 2020; 1248: 33-59.
- Kuol N, Stojanovska L, Nurgali K, Apostolopoulos V et al. PD-1/PDL1in disease. Immunotherapy. 2018; 10: 149-160.
- Ghosh C, Luong G, Sun Y. A snapshot of the PD-1/PD-L1pathway. J
Cancer. 2021; 12: 2735-2746.
- Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1
and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations.
J Immunother Cancer. 2018; 6: 8.
- Khan M, Zhao Z, Arooj S, Fu Y, Liao G. Soluble PD-1: Predictive, Prognostic, and Therapeutic Value for Cancer Immunotherapy. Front
Immunol. 2020; 11: 587460.
- Gurram RK, J Zhu. Orchestration between ILC2s and Th2 cells in
shaping type 2 immune responses. Cell Mol Immunol. 2019; 16:
225-235.
- Wan J, Cai W, Wang H, Cheng J, Su Z, et al., Role of type2 innate
lymphoid cell and its related cytokines in tumor immunity. J Cell
Physiol. 2020; 235: 3249-3257.
- Ercolano G, Falquet M, Vanoni G, Trabanelli S, Jandus C, et al.
ILC2s: New Actors in Tumor Immunity. Front Immunol. 2019; 10:
2801.
- Bruchard M, Ghiringhelli F. ILC2s in cancer: context matters. Nat
Immunol. 2021; 22: 804-806.