Introduction
Metastatic involvement of the skull base is a well-known complication of a variety of systemic cancers. In these cases, the clinical diagnosis is usually suspected by the presence of symptoms
and signs secondary to the involvement of cranial nerves that exit
through the basal foramina, together with persistent headache.
The orbital, parasellar, middle fossa, jugular foramen and Occipital Condyle Syndrome (OCS) were the five clinical syndromes
associated with skull base metastases described by Greenberg et
al in 1981[1]. The OCS is characterized by unilateral occipital pain
and unilateral tongue paralysis. Although a group of patients with
OCS have a benign explanation, such as trauma, infection, stroke
and Guillain-Barré syndrome, a wide variety of malignancies account for the remainder of cases of OCS. In fact, in these cases, the OCS is usually the first clinical manifestation of the neoplasm.
We report the first case of OCS associated with a malignant insulinoma.
Case report
This 57 year-old woman began in 2005 with recurrent neurological symptoms associated with hypoglycemia. A CT scan demonstrated a pancreatic mass. She underwent distal pancreatectomy
and splenectomy, and the histopathological examination of the
excised tissue revealed a pancreatic insulinoma. At that time, hepatic islet cell metastases were observed. The liver metastases
had no change after two chemoembolizations with polyvinyl alcohol particles and treatment with octreotide and diazoxide was administered. In 2009, she underwent liver transplantation for the
metastatic neuroendocrine tumour with no incidences. She was on tacrolimus, which was changed to everolimus one year later.
Two years after liver transplantation, a CT scan of the abdomen
disclosed peritoneal, lymph node and hepatic metastases and capecitabine was added. Five days before his admission in February
2010, the patient began to have dysarthria, dysphagia, and progressive left occipital pain. He complained of severe, continuous,
left occipital pain that became unbearable with left suboccipital
palpation or on neck rotation to the right. Routine analgesics were
of no help and morphine was administered to control pain. There
were no cervical masses on palpation. Neurological abnormalities
were limited to left hypoglossal nerve paralysis. An osteolytic lesion was found in the left clavicle by a thoracic CT examination. An
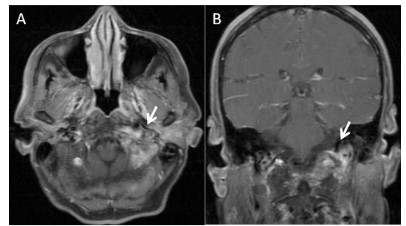
MRI study of the skull base showed an enhancing soft tissue mass
close to the left foramen magnum without intracranial invasion
(Figure 1). A brain MRI was found to be normal. His general status
deteriorated, and the patient died one week later.
Discussion
Insulinoma is a rare neuroendocrine tumour with an annual
incidence of 0.4 cases per 100 000 people. Insulinoma has malignant characteristics defined by metastases in only 10% of the
cases [2,3]. Most patients with malignant insulinoma have lymph
node or liver metastases and only rarely involve other organs,
such the skeletal system. The available treatments show only
short-term benefits and the prognosis of these patients is relatively poor with a median survival period of approximately 2 years
[2]. Insulinoma could be a feature of multiple endocrine neoplasia
type 1 (MEN1) and approximately 4% of patients with insulinoma
will have MEN1; in this case is very unlike a MEN1-associated insulinoma based on the absence of a family history of MEN 1 and
the absence of other clinical, biochemical or radiological characteristics of MEN 1 [4]. Due to the small number of patients with
malignant insulinoma, there are little data regarding its neurological complications. Neurological symptoms are frequent in the
initial phases of insulinoma. Hypoglycemia due to insulinomas mimics a great variety of neurological conditions and most patients
with present with neurological or psychiatric manifestations that
often lead to misdiagnosis. Symptoms from central nervous system glucose deprivation could persist for years since the diagnosis due to unregulated secretion of insulin and proinsulin-related
products from malignant insulinoma [5,6]. In addition, peripheral
neuropathy is unusually reported as a neurological complication
in the course of insulinoma probably due to maintained hypoglycemia rather than secondary to hyperinsulinaemia [7]. To our
knowledge, no other neurological features of insulinoma have been reported except symptoms due to hypoglycemia of either
the central or peripheral nervous system.
The hypoglossal nerve arises from the motor nucleus located
beneath the floor of the fourth ventricle, passing in front of the
vertebral and posterior inferior cerebellar arteries. It exits the
base of the skull through the hypoglossal canal in the occipital
bone; it then traverses the neck and curves back, divides and innervates the tongue muscles. Metastatic involvement of the occipital condyle originates the OCS, which is characterized by unbearable unilateral occipital pain and ipsilateral tongue paralysis.
Several malignant causes have been described as a cause of OCS,
including metastases from different types of tumours [1,8-15].
but we have found no reference in the English language literature
to isolated hypoglossal neuropathy caused by insulinoma metastases to the base of the skull.
When OCS is suspected based on its stereotyped symptoms, CT
and MRI are the diagnostic steps. The MRI is better for delineating
soft tissues. The sections of CT and MRI must be low enough to
show the foramen magnum area to detect the suspected lesion.
In patients with skull base metastases known to have untreatable
cancer, local palliative radiation therapy should be undertaken
as soon as possible [16]. When this treatment is delivered early,
symptomatic relief can be expected in most patients. In patients
without known systemic cancer, skull base metastases can be the
first manifestation of neoplasia and a search for a primary source
is indicated in patients with OCS.
Conclusion
In conclusion, we added malignant insulinoma as a cause of
OCS; clinicians should keep in mind the diagnosis of OCS even in
tumours for which OCS has not been reported in the literature. In
addition, this description of malignant insulinoma contributes to
a better understanding of its clinical course to clarify the role of
therapeutic procedures in the disease.
References
- Greenberg HS, Deck MD, Vikram B, Chu FC, Posner JB. Metastasis
to the base of the skull: clinical findings in 43 patients. Neurology.
1981; 31: 530-537.
- Mathur A, Gorden P, Libutti SK. Insulinoma. Surg Clin North Am.
2009; 89: 1105-1121.
- Sada A, Glasgow AE, Vella A, Thompson GB, McKenzie TJ, et al. Malignant Insulinoma: A Rare Form of Neuroendocrine Tumor. World
J Surg. 2020; 44: 2288-2294.
- Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H,et al. Clinical practice guidelines for multiple endocrine neoplasia type 1
(MEN1). J Clin Endocrinol Metab. 2012; 97: 2990-3011.
- Ding Y, Wang S, Liu J, Yang Y, Liu Z, et al. Neuropsychiatric profiles
of patients with insulinomas. Eur Neurol. 2010; 63: 48-51.
- Shaw C, Haas L, Miller D, Delahunt J. A case report of paroxysmal
dystonic choreoathetosis due to hypoglycaemia induced by an insulinoma. J Neurol Neurosurg Psychiatry. 1996; 61: 194-195.
- Tintoré M, Montalbán J, Cervera C, et al. Peripheral neuropathy
in association with insulinoma: clinical features and neuropathology of a new case. J Neurol Neurosurg Psychiatry. 1994; 57: 1009-
1010.
- Takeuchi S, Osada H, Nagatani K, Shima K. Occipital condyle syndrome as the first sign of skull metastasis from lung cancer. Asian J
Neurosurg. 2017; 12: 145-146.
- Marruecos J, Conill C, Valduvieco I, Vargas M, Berenguer J, et al.
Occipital condyle syndrome secondary to bone metastases from
rectal cancer. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl
Cancer Inst Mex. 2008; 10: 58-60.
- Capobianco DJ, Brazis PW, Rubino FA, Dalton JN. Occipital condyle
syndrome. Headache. 2002; 42: 142-146.
- Morís G, Roig C, Misiego M, Alvarez A, Berciano J, et al. The distinctive headache of the occipital condyle syndrome: a report of four
cases. Headache. 1998; 38: 308-311.
- Moeller JJ, Shivakumar S, Davis M, Maxner CE. Occipital condyle
syndrome as the first sign of metastatic cancer. Can J Neurol Sci J
Can Sci Neurol. 2007; 34: 456-459.
- Rodríguez-Pardo J, Lara-Lara M, Sanz-Cuesta BE, Fuentes B, DíezTejedor E. Occipital Condyle Syndrome: A Red Flag for Malignancy.
Comprehensive Literature Review and New Case Report. Headache. 2017; 57: 699-708.
- Salamanca JIM, Murrieta C, Jara J, Munoz-Blanco JL, Alvarez F, et
al. Occipital condyle syndrome guiding diagnosis to metastatic
prostate cancer. Int J Urol. 2006; 13: 1022-1024.
- Saraswat MK, Perera RW, Renwick I, Zuromskis T, Singh V, et al.
Occipital condyle syndrome: self diagnosed. BMJ Case Rep. 2009;
2009: bcr08.2008.0636.
- Rades D, Schild SE, Abrahm JL. Treatment of painful bone metastases. Nat Rev Clin Oncol. 2010; 7: 220-229.