Introduction
Colorectal Cancer (CRC) is one of the most common gastrointestinal malignancies worldwide, with the third-highest rate
of occurrence and the second-highest rate of mortality of all cancers [1,2]. Recurrence and metastasis are the principal reasons
for CRC treatment failure; the 5-year survival rate of patients
with metastasis is only 10% [3]. An international study found that
more than 25% of patients with stage III colon cancer experienced
recurrence or metastasis within 3 years of surgery [4]. The median Disease-Free Survival (DFS) of patients with stage III CRC is
19 months [5]. Therefore, the prevention of postoperative recurrence or metastasis of stage III 61 CRC remains an urgent clinical problem. According to the National Comprehensive Cancer
Network® guidelines, stage III CRC treatment is based on surgical
procedures, with radiotherapy and chemotherapy as postoperative adjuvant treatment [6]. Adjuvant chemotherapy has become
a routine treatment for stage III colorectal cancer in China and
worldwide [7]; however, the curative effect of these treatments
remains unfavorable, and the efficacy of 5-flurouracil-based therapy is frequently compromised by the development of chemotherapy resistance [8]. Recently, there has been an increase in
clinical research on Chinese Herbal Medicine (CHM) for CRC. Recent studies found that CHM can be administered as an effective
auxiliary therapy to significantly improve the quality of life and
reduce the adverse reactions to chemotherapy in patients with
CRC [9-11]. In addition to adjuvant chemotherapy, many Chinese
patients also use CHM to delay CRC recurrence [12-14]. A prospective multicentre study confirmed that a longer duration of
CHM use significantly improved the survival outcomes of patients
with stage II–III CRC in Beijing, China [12]. Additionally, a randomised, double-blind, placebo-controlled clinical trial in Shanghai,
Nanjing, and the Central Plains of China reported that PRM1201
improved the adjuvant treatment of stage III colon cancer [14].
Therefore, concomitant with the ever-increasing categorical evidence-based pharmacological therapies, the clinical utilisation of
CHM has proven to be a valuable approach for treating CRC.CHM
is deeply rooted in Chinese culture, especially in the Guangdong
province, where it has been used for thousands of years as the
preferred method of treating diseases [15]. However, CHM use
varies across regions. For example, the climate in southern China
is humid, which is significantly different from that of the northern
region, and, thus, treatment concepts and curative effects are
specific and based on the characteristics of Lingnan CHM. According to pre-clinical data in one report, in the classification of
patients for stage III CRC admission, colonic damp-heat and stasis-toxin syndrome was observed in 60% of patients, for whom
the indicated Traditional Chinese Medicine (TCM) treatment is
blood stasis removal, detoxification, and clearing of heat [15,16].
A previous study showed that the Qu-Yu-Jie-Du (QYJD) CHM therapy was related to improved survival outcomes in CRC patients
with liver-limited metastases [17], preliminarily confirming that
QYJD decoction may inhibit intestinal cancer cells by activating
the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway through the epigenetic regulation of demethylation [18].
In Guangdong province, the effects of QYJD decoction on the survival outcomes of patients with stage III CRC remain unknown.
Hence, we conducted this study to clarify whether QYJD decoction is associated with a reduced risk of recurrence in patients
with stage III CRC and clarified the mechanism by which QYJD decoction delays tumour occurrence and development through
network pharmacology and animal experiments.
Materials and methods
Study participants: The retrospective cohort study was
conducted at our hospital in China.
Inclusion and exclusion criteria: Patients diagnosed with stage
III CRC pathologically or clinically, who had undergone radical surgery and were admitted to our hospital between 2016 and 2018
were included in our study. Patients were excluded if they had
been diagnosed with secondary CRC; had additional organ malignancies; had received neoadjuvantchemotherapy, radiotherapy,
or targeted therapy; had pathological tumour classification or clinical characteristics that were severely fragmented, or presented
with severe primary diseases, such as heart, kidney, liver diseases;
or had a DFS period of less than 3 months.
Ethics: Inclusion and exclusion criteria: Patients diagnosed with stage
This study was approved by the ethics committee of
our hospital, and the need for informed consent was waived. The
research was conducted in accordance with the World Medical
Association Declaration of Helsinki.
Exposure: CHM therapy use Patients were assigned to exposure groups according to the literature; those who had taken CHM
for more than 3 months during the study period were allocated to
the Chinese medicine treatment group (CHM intake group), and
other patients were assigned to the group that did not take CHM
(non-CHM intake group). Based on prognostic follow-up data, we
also calculated the duration of CHM therapy received by classifying TCM users into two groups as follows: a regular Chinese
medicine group comprising patients who took Chinese medicine
regularly (at least 14 doses per month) for at least 12 months and
an intermittent CHM group comprising those who took CHM irregularly (less than 14 doses per month) for between 3 months
and 1 year.
Covariates: In the retrospective cohort study, we recorded
information, including sex, age, pathological tumour type, clinical TNM staging, tumour differentiation, anatomical location of
the tumour, adjuvant radiotherapy, and adjuvant chemotherapy.
According to previous studies, we collected variables that have a
known impact on the prognosis of stage III CRC, including Right-Sided Colon Cancer (RCC) and Left-Sided Colon Cancer (LCRC);
intravascular tumour thrombus; nerve bundle infiltration; and
unstable microsatellites. The chemotherapy regimens used for
the enrolled patients were as follows: oxaliplatin + leucovorin +
fluorouracil, oxaliplatin + capecitabine, and other combinations.
The ancient and modern medical record cloud platform software
Preclinical data statistics show that blood stasis removal and detoxification is the basic treatment method. We collected the available TCM prescriptions and input the data into a cloud platform
software of ancient and modern medical records. A database of
patients with stage III CRC was established; the data were standardised and added to the analysis pool for data mining analysis.
Moreover, we used binomial association rule and cluster analyses
to conduct data mining on the frequency of use of high-frequency
drugs with the accumulation of stasis and toxins.
Therapeutic efficacy evaluation
DFS time, defined as the time from the start of the treatment to the observation of metastasis/recurrence or death, was the main
endpoint indicator for evaluating the survival outcome of patients
with CRC [5]. Data were acquired by the hospital medical record
system or telephone follow-up. DFS time was calculated in months,
and the assessors were blinded to patient information to remove
assessment bias. All data were updated on August 30, 2021.
Cell culture
Human LoVo CRC cells were purchased from the Cell Bank
of the Chinese Academy of Sciences in Shanghai, and cells were
cultured in RPMI-1640 complete medium containing 10% fetal
bovine serum and incubated at 37oC and 5% CO2. LoVo cells were
seeded into mouse 184 coecal sub mucosal tissue at a concentration of 5 × 104/mL in a volume of 10–15 μL.
Animal source
BALB/c-nu nude female mice, SPF grade, weighing 18~22
g, were purchased from Nanjing University-Nanjing Institute
of Biomedicine (laboratory animal certificate number: NO.
32002100007118). The experimental animals were kept in the
SPF animal room of Guangdong Pharmaceutical University (experimental unit license number: SYXK(Guangdong 2014-0125) and
randomly grouped in individual experiments (n = 6 per group).
Experimental drug
(1) QYJD decoction: The prescription for removing blood stasis
and detoxification comprises Coix seed, Burnet, Sophora japonica,
peach kernel, and turtle bug, among others. All CHM in the recipe
were purchased from the Chinese Pharmacy of the First Affiliated
Hospital of Guangzhou University of Chinese Medicine. Using the
body surface area (BSA) method, we calculated the daily dose of
21.6 g/kg for the 203 mice (equivalent to the adult daily dose).
(2) Acetylsalicylic acid (ASA, Aspirin): Purchased from the First
Affiliated Hospital of Guangzhou University of Chinese Medicine,
was dissolved in water (2 mg/mL) at a dose of 20 mg/kg/day for
the experimental mice (corresponding to a daily dose of 150 mg
for an adult according to the BSA method).
Coecal xenograft mouse models in an enteritis-simulated environment. Acute enteritis was induced by administering 1.5% dextran sulfate sodium salt (DSS) for 1 week, simultaneous with the
initiation of the experiment of the drug treatment group. During
the period, the disease index was scored according to the weight
of mice, stool characteristics, and presence of blood in stool; 1–3
days after the end of DSS administration, LoVo cells were planted
in the submucosa of the coecum, and the drug was continued for
2 weeks. After the treatment, mice in each group were sacrificed
after sodium pentobarbital-induced anaesthesia; the entire colon
was dissected, the contents were taken out, the colon length was
measured, and the tumour volume was calculated.
Immunohistochemistry assay
The tumour tissues were sliced into 4–6 μm sections, and antigens were retrieved after deparaffinisation with graded alcohol.
Tissue sections were subsequently blocked with 5% bovine serum
albumin for 60 min. Tissue sections were incubated with anti-ki67
(1:100) antibodies at 4oC overnight. The secondary antibody was
added, and the resulting mixture was incubated at room temperature for 1 h after washing three times. Subsequently, diaminobenzidine solution was used for staining; the sections were counterstained with hematoxylin, dehydrated, and mounted on slides.
Sections showing brownish yellow under the microscope indicated positive results.
Network pharmacology analysis
Based on the network pharmacology ‘disease-gene-target-drug’ interaction network, the pharmacokinetic parameters oral
availability ≥30% and drug similarity ≥0.18 in the Traditional
Chinese Medicine Systems Pharmacology Technology Platform
(TCMSP) and a Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine (BATMAN-TCM) were used
to screen the potential 241 active ingredients of QYJD decoction.
A score cutoff ≥20 (P≤0.05) was used to predict and screen the
corresponding targets. With a score ≥5 as the screening criterion,
the target genes related to CRC were retrieved from the Gene
cards database, and the common targets were screened by intersecting with the active ingredient target genes in QYJD decoction.
The String database and Cytoscape were used to build a ‘component-target’ network. Using the database for annotation, visualisation, and integrated discovery (DAVID) database, Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and
Genomes (KEGG) metabolic pathway analysis were performed on
the common targets of the QYJD decoction and CRC.
Statistical analysis
Baseline differences between the CHM and non-CHM intake
groups were compared. Data were presented as means ± standard deviation for continuous variables and as frequencies (percentages) for categorical variables according to CHM intake. Three
variables, including intravascular cancer thrombus, bundle invasion, and microsatellite state showed a high missing rate. We used
the multiple imputation function of missing data to improve the
missing data of the three sets of variables. One-way analysis of
variance (ANOVA), Kruskal-Wallis H tests, and chi-squared tests
were employed to evaluate any statistical differences between
groups. The association between CHM therapy and DFS in stage
III CRC patients was determined using a univariate linear regression model and multivariate-adjusted models. Considering the
unbalanced baseline between the CHM and non-CHM groups, we
conducted exploratory subgroup analyses and interaction detection to test the stability of the relationship. The mean ± standard
error (x ± s) of the experimental data is expressed. For normally
distributed continuous data, one-way ANOVA was used for comparison among groups. Otherwise, the Kruskal-Wallis H test was
used for comparison among groups. If the difference between
groups was statistically significant, the Student-Newman-Keuls
method, Bonferroni method, or the Dwas, Steel, Critchlow Flinger method was used for multiple comparisons. For repeated
data at different time points in three or more groups, such as the
body weight of mice and disease activity index (DAI) indicators,
repeated measures analysis of variance was used. Analyses were
performed using the statistical software packages R and Empower
States. All tests were two-sided, and the statistical significance level was set at 0.05.
Results
In our study, the high-frequency medicines for removing blood
stasis and detoxification were extracted from all Chinese medicine prescriptions in the retrospective cohort study, which almost
coincides with QYJD decoction. Animal experiments were used to
confirm the anti-inflammatory and tumour-inhibiting effects of
QYJD decoction. The main active components and action pathways
of QYJD 291 decoction were speculated based on network pharmacology. The flow diagram of the present study is shown in Figure 1.
Patients
By July 30, 2018, 206 medical records were collected; 177 patients met the inclusion criteria, whereas 40 were excluded (Figure 2A). Of these, 121 (69.94%) patients received TCM therapy,
64 were taking Chinese medicine intermittently, and 57 were taking Chinese medicine regularly. After August 2020, those whose
information had not been reviewed through telephone follow-up
or the electronic medical record system were considered lost to
follow-up. In this study, 13 patients were lost to follow-up, with
a loss follow-up ratio of 7.34%. Information on the DFS of 111
(62.71%) and 53 (29.94%) patients was collected via the hospital
medical record system and telephone follow-up, respectively.
Participant characteristics
The study participants were enrolled from January 5, 2016 to
June 25, 2018. The median follow-up time was 51.07 months;
however, the median DFS could not be determined; the average DFS time was 34.87 ± 19.59 months. Of the 173 patients,
69 (42.07%) experienced recurrence or metastasis. The demographic and clinical characteristics of the study patients classified by
CHM use are presented in Table 1. As shown in Table 1, the two
groups were unevenly distributed with regard to factors such as
age, bundle invasion, adjuvant chemotherapy, and complete adjuvant chemotherapy cycle. Moreover, a higher proportion of patients received chemotherapy (75.21% vs. 59.62%) and completed chemotherapy (57.26% vs. 40.00%) in the CHM intake group
than in the CHM intake group. Baseline characteristics and clinical features classified by CHM intake (none, intermittent, or regular CHM intake) showed that age (P=0.075), T stage (P=0.088),
bundle invasion (P=0.099), and adjuvant chemotherapy (P=0.036)
were unevenly distributed.
CHM therapy and DFS
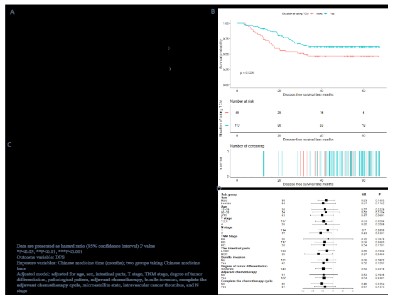
The median survival and hazard ratio (HR) of mortality for each
subgroup are shown in Figure 2. The predefined primary end-point in our analysis was the DFS. The median DFS for the CHM
intake group 333 could not be determined; however, it was 31.67
months in the non-CHM intake group. The 1-, 2-, and 3-year recurrence and metastasis rates in the CHM intake group were 7.96%,
24.29%, and 38.21%, respectively, while those in the non-CHM
intake group were 20.41%, 337 46.94%, and 53.52%, respectively
(Figure 2B). Univariate Cox proportional-hazards regression model analysis revealed that the CHM intake group showed a no-table 43% decrease in recurrence/metastasis rate (HR = 0.570;
95% confidence interval [CI], 0.35–0.94, P=0.0266). In particular, on applying the Cox proportional-hazards regression model
to adjust covariates such as age, bundle invasion, adjuvant chemotherapy, completion of the chemotherapy cycle, and so on,
the HR was 0.48 (95% CI, 0.27–0.87; 345 P=0.0147). Moreover,
relative to the non-CHM intake group, the multivariate Cox proportional-hazard regression model analysis result showed that
the risk of recurrence/metastasis in the intermittent CHM intake
group was significantly reduced by 31% (HR=0.69, P=0.2416) and
it was decreased by 73% (HR=0.27, P=0.0019) in the regular 350
CHM intake group. Considering the baseline differences between
the two groups, we further conducted stratified analysis and
statistical interaction methods to confirm the influence of CHM
on the prognosis of stage III 354 CRC patients. We examined the
effect of CHM on DFS in patients in different variable stratifications (Figure 2C). The association between CHM intake and DFS in
patient outcomes seemed consistently stratified by 358 age, sex,
tumour characteristics, and different treatments. The relationship
between enteritis and DFS in stage III CRC patients. The univariate
Cox regression model showed that stage III CRC patients with a
history of colitis had a 1.45-fold increased risk of recurrence and
metastasis (HR=2.45; 95% CI, 1.14–5.25; P=0.0218). The multivariate Cox regression models suggested a 2.08-fold increased
risk of recurrence and metastasis (HR=3.08; 95% CI, 0.95–10.02;
P=0.0613).
QYJD decoction and stage III CRC patients
Even more, in the univariate analysis, we found that the syndrome of heat-toxin and blood stasis can increase the risk of
recurrence and metastasis in patients with stage III CRC by 1.37
times (HR=2.37; 95% 372 CI, 1.07–5.23; P=0.0332). The syndrome
of heat-toxin and blood stasis is one of the main syndrome types
of colorectal cancer, which is generally treated with the QYJD decoction. In order to further confirm the high-frequency TCM combination for stage III CRC stasis and toxin accumulation syndrome,
a total of 416 CHM prescriptions for the large intestine stasis-toxin
syndrome (stasis-toxin accumulation) were analysed. Among
them, there were 324 prescriptions for simple TCM maintenance
treatment and 92 prescriptions for adjuvant chemotherapy. The
statistics of the 92 prescriptions for the stasis-toxin accumulation
syndrome type involved a total of 166 TCMs. The frequency of all
medicines was counted. Furthermore, 10 medicines with a frequency of use of ≥50% are listed in descending order of frequency
as follows: Semen persicae, Ground beetle, Radix sophoraefalvescentis, Radix Sanguisorbae, Solanum nigrum, Herba Sarcandrae,
Shancigu, Pachymacocos, Atractylodes, and Taxus chinensis.We
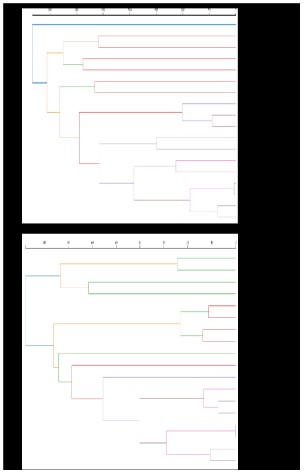
used cluster analysis to screen out 18 TCMs that were used more
than 100 times, which almost coincides with QYJD decoction.The
ranking results are shown in Figure 3A.
Clinically, apart from the method of removing blood stasis and
detoxification being useful in attacking cancer pathogens, it also
has great importance in strengthening the spleen, nourishing qi,
and protecting stomach qi. Especially during adjuvant chemotherapy, the frequency of use of ChenxiaSijunzi decoction was significantly higher than that of TCM maintenance therapy (Figure 3B).
QYJD decoction inhibits enteritis symptoms in mice with coecaltumours in an enteritis-simulated environment Repeated measures
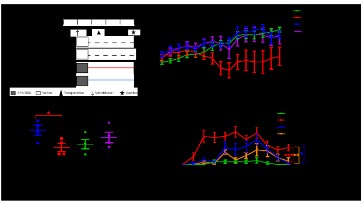
ANOVA indicated that there was a significant difference in body
weight between different groups (P=0.001), and there was a significant interaction between body weight and time (Greenhouse-Geisser test, P=0.017) (Figure 4B). There were differences in the
body weight of mice between groups at different experimental
times. From the 10th day of the experiment, the model group
mice body weight significantly decreased (P<0.1), whereas that of
mice treated with QYJD decoction and ASA significantly increased
(P<0.1).
QYJD decoction inhibits inflammatory symptoms in mice with
intestinal cancer in an enteritis-simulated environment. We measured the colon length (the length from the coecum to the anus)
of mice in each group: compared with the control group, the
colon length of mice in the model group was significantly shorter (P=0.024). Compared with the model group, colon length of
the QYJD group was longer but the difference was not significant
(P=0.16) (Figure 4C). After the mice in each group were orally
administered DSS, the body weight, stool quality, and presence
of blood in stool were monitored daily. DAI was assessed in all
groups. Repeated-measures ANOVA showed that there was a
significant difference in DAI value between different groups
(P=0.02), and there was a significant interaction between DAI
value and time (Greenhouse-Geisser test, P=0.000). Compared
with mice in the blank group, the DAI of mice in the model group
changed significantly (P=0.000), and this change became apparent from the 4th day, reached the maximum on the 7th day, and
lasted for 21 days. Compared with mice in the model group, the
DAI values of the mice in the QYJD decoction group (P=0.002) and
ASA (P=0.022) group were significantly lower (Figure 4D).
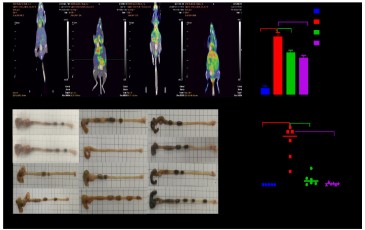
QYJD decoction inhibits tumour proliferation in mice with coecaltumours in an enteritis-simulated environment. The influence
of QJYD decoction on the inhibition of tumours in mice: QYJD decoction can reduce the glucose metabolism in the tumour. After
21 days of administration, 3 mice in each group were randomly
selected for positron emission tomography/computed tomography. The level of SUVmax could reflect the uptake of glucose metabolism by the lesions; the SUVmax value of the model group
was significantly higher than that of the control group (P=0.000);
compared with the model group, the SUVmax value of the QYJD
decoction group P=0.001) and the ASA group (P=0.005) decreased
significantly. The effect of QYJD decoction on the volume of tumours in the intestinal cancer mouse model: The colon tumour
volume of mice in the model group was significantly larger than
that of normal mice (P=0.012). Compared with the model group,
the tumour volumes of mice in the QYJD group (P=0.013) and ASA
group (P=0.015) were significantly reduced.
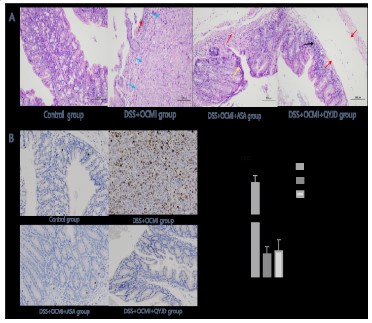
The expression of Ki67 in the intestinal tract of mice in the model group and the QYJD decoction group Ki-67 is a nuclear antigen
related to cell proliferation, which only exists in cells in the proliferation cycle. The Ki67 value of the model group was significantly
increased compared with that of the normal group; additionally,
the QYJD decoction and aspirin groups had significantly reduced
expression of tumour tissue growth-related protein Ki67 compared with the model group (Figure 6B).
QYJD decoction & CRC network pharmacological analysis. With
the limited conditions, there were 23 active components and 95
target proteins in peach kernel; 1 active component and 32 target
proteins in P. chinensis; 8 active components and 122 target proteins in dandelion; 42 active components and 971 target proteins
in Sophoraflavescens; 15 in Tuckahoe 1 active ingredients and 222
target proteins, 9 active ingredients and 48 target proteins of Coix
seed, 9 active ingredients and 496 targets of Burnet, 6 active ingredients and 366 targets of Sophora japonica. After removing the
common targets, we finally got a total of 1,411 component targets. A total of 8,389 CRC-related targets were collected from the
Genecards database, and a total of 2,740 CRC target genes were
collected with a score ≥5 as the screening condition. This intersected with the 1,411 targets corresponding to the active ingredients
of the QYJD decoction, and we obtained 518 common targets,
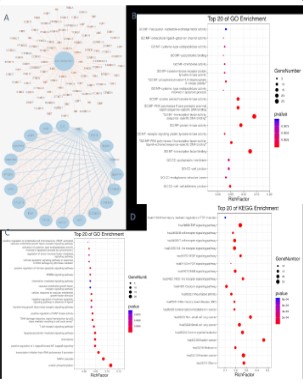
which were the predicted targets for the anti-colorectal cancer effect of the QYJD decoction. We imported the target interaction relationship obtained from the STRING database into the Cytoscape
3.6.0 software and selected all 105 target genes and the top 20
target genes that meet the card value for visualisation, as shown
in Figure 7A. TP53, AKT1, INS, IL-6, EGFR, ESR1, MAPK3, MAPK1,
SRC, STAT3, JUN, TNF, PTEN, CTNNB1, CCND1, IGF1, EP300, FOS,
AR, CREB1, IL1B, MTOR, CXCR4, and PIK3R1 are all potential targets for the treatment of CRC with QYJD decoction.
GO enrichment and KEGG pathway enrichment analyses were
performed on the targets of QYJD decoction against CRC by the
DAVID database. The GO enrichment analysis results showed that
the enriched genes satisfying molecular function analysis P<0.01
were included in cadherin binding involved in cell adhesion, chemokine activity, apoptosis-related cysteine-type endopeptidase
activity, protein serine/threonine kinase activity, and transcription
factor binding. A large number of enriched genes satisfying cell
composition analysis P<0.01 were associated with the postsynaptic membrane, cell junction, endoplasmic reticulum, and cell-cell
adherens junction, which are closely related to the mechanism
of CRC occurrence and development (Figure 7B). In the biological process analysis of cell components, the genes that satisfy
P<0.01 and have a large number of enriched genes were involved
in apoptosis, chemokines, protein phosphorylation, vascular endothelial growth factor receptor signaling pathway, and I-kappa
B positive regulation of kinase/NF-kappa B signaling, cell cycle
arrest, positive regulation of MAP kinase activity, transforming
growth factor beta-receptor signaling, P53-mediated cell cycle arrest signaling, extra cellularity in the absence of ligands, and negative regulation of 515 the apoptotic signaling pathway (Figure 7C).
Based on the KEGG pathway enrichment analysis, we speculated
that tumour choline metabolism, regulation of Transient receptor
potential channels by inflammatory mediators, tumour necrosis
factor signaling pathway, B cell receptor signaling pathway, T cell
receptor signaling pathway, toll-like receptor signaling pathway,
VEGF signaling pathway, mTOR signaling pathway, ErbB signaling
pathway, and NOD-like receptor signaling pathway, among other
pathways, lay a primary role in the treatment of CRC (Figure 7D).
Discussion
This is a study on the clinical application and formation of empirical prescriptions. From basic experiments to clinical research,
we have repeatedly demonstrated that QYJD decoction may inhibit the occurrence and development of tumours by targeting anti-inflammatory pathways. The data in this study were collected from the CRC database of the Cancer Center in our hospital.
Patients in this database were followed up regularly. The data co-variates of this study were comprehensively collected; the data
were true and reliable, the loss to follow-up rate was low, and
the data quality was high. To avoid confounding factors affecting
the core outcome, we collected literature reports on confounding
factors related to the prognosis of CRC, such as the left and right
hemiguts [19], stability of MSI [20], and the presence of vascular
invasion. In this study, we found a notable decrease in recurrence
and metastasis risk in patients who received CHM therapy for
over 3 months, indicating that longer-lasting CHM intake correlated with better DFS. In addition, the results of univariate and
multivariate analyses suggest that inflammatory bowel disease is
an independent risk factor for recurrence and metastasis in patients with stage III CRC after radical resection. Further, the stasis-toxin accumulation syndrome type is a risk factor for stage III
CRC. The top 18 Chinese medicines in the prescriptions of stasis
and poison accumulation syndrome include QYJD decoction except for CHM Tufuling (Smilax 550 glabraRoxb.). Previous research
results have confirmed that QYJD decoction has an evident anti-inflammatory effect, 18 Inflammation-cancer transformation
is one of the classic pathways of CRC [21-23], and inflammation related biomarkers may be used to predict the prognosis of colorectal cancer [24]. Inflammation can promote cell proliferation,
inhibit apoptosis, recruit immunocytes or enhance cytoskeletal
remodeling by microRNA signaling, affect genome stability, and
lead to tumourformation [25,26]. Gut microbiota dysbios is alters
mitochondrial metabolism in mucosal cells, induces activation of
inflammasome signaling pathways, and alters epithelial barrier
function [27]. In addition, there is increasing evidence that inflammation interacts with epithelial-mesenchymal transition and
cancer stem cells. With the malignant initiation of epithelial cells,
chronic inflammation further maintains a pro-inflammatory and
tumour565 promoting microenvironment, mediating tumour progression and spread [28]. Studies have found that the NF-kappa
B (NF-κB) signaling pathway plays an extremely important role in
the maintenance of chronic inflammation and is an important mediator of epithelial cell growth and deterioration in a pro-inflammatory environment [29,30]. The TLR4/NF-κB signaling pathway
or the PI3K/AKT/NF-κB signaling pathway is activated in colon cancer, causing the production of IL-6 and TNF-α, as well as tumour
growth and metastasis [31,32]. Therefore, we speculate that QYJD
decoction could inhibit the NF-kB inflammatory pathway in CRC,
as demonstrated by network pharmacology. The prevention of
recurrence after radical resection for CRC conforms to the characteristic of ‘healing and preventing recovery,’ which is an aspect
of TCM treatment. It is also the entry point for the use of CHM
to treat tumours. The core study results preliminarily confirmed
the clinical application and mechanism of action of the method
of removing blood stasis and detoxification in the Lingnan region
of China after surgery for stage III CRC. This provides a new possibility for clinical adjuvant therapy after CRC surgery. However,
as a single-centre retrospective cohort study, our study also had
certain limitations. The level of evidence is not sufficiently high,
and the mechanism of action of the inflammatory pathway is not
proven. Therefore, future multi-centre prospective cohort studies
and basic experimental verification studies in the Lingnan Regional TCM Oncology Center are required.
Conclusions
This study confirms that removing blood stasis and detoxification can reduce postoperative recurrence or metastasis of stage III
CRC, and its mechanism may involve the inhibition of the inflammatory pathway.
Abbreviations
ANOVA: Analysis Of Variance; BSA: Body Surface Area; CHM:
Chinese Herbal Medicine; CRC: Colorectal Cancer; DAI: Disease
Activity Index; DAVID: Database For Annotation, Visualization, and
Integrated Discovery; DFS: Disease-Free Survival; DSS: Dextran
Sulfate Sodium salt; GO: Gene Ontology; HR: Hazard Ratio; KEGG:
Kyoto Encyclopedia of Genes and Genomes; LCRC: left-sided colon cancer; QYJD: Qu-Yu-Jie-Du; RCC: Right-Sided Colon Cancer.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J
Clin. 2019; 69: 7-34.
- Jiang Y, Yuan H, Li Z, Ji X, Shen Q, et al. Global pattern and trends
of colorectal cancer survival: a systematic review of population-based registration data. Cancer Biol Med. 2021; 19: 17 5-186.
- Labianca R, Merelli B. Screening and diagnosis for colorectal cancer: present and future. Tumori. 2010; 96: 889-901.
- Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018; 378:
1177-1188.
- Sargent D, Shi Q, Yothers G, Yoshino T, Paul J, et al. Two or three
year disease free survival (DFS) as a primary end-point in stage
III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12, patients from MO-SAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer. 2011;
47: 990-996.
- Tsikitis VL, Larson DW, Huebner M, Lohse CM, Thompson PA. Predictors of recurrence free survival for patients with stage II and III
colon cancer. BMC Cancer. 2014; 14: 336.
- Chapuis PH, Bokey E, Chan C, Wu M-Y, Sun M-F, et al. Recurrence
and cancer specific death after adjuvant chemotherapy for Stage
III colon cancer. Colorectal Dis. 2019; 21: 164-173.
- Taieb J, Gallois C. Adjuvant chemotherapy for stage III colon cancer. Cancers (Basel). 2020; 12: 2679.
- Kuo YT, Chang TT, Muo CH, Wu M-Y, Sun M-F, et al. Use of complementary traditional Chinese medicines by adult cancerpatients in
Taiwan: A nationwide population-based study. Integr Cancer Ther.
2018; 17: 531-541.
- Kong MY, Li LY, Lou YM, Chi HY, Wu JJ. Chinese herbal medicines
for prevention and treatment of colorectal cancer: From molecular mechanisms to potential clinical applications. J Integr Med.
2020;18: 369-384.
- Sun Q, He M, Zhang M, Zeng S, Chen L, et al. Traditional Chinese
medicine and colorectal cancer: Implications for drug discovery.
Front Pharmacol. 2021; 12: 685002.
- Xu Y, Mao JJ, Sun L, Yang L, Li J, et al. Association between use
of traditional Chinese medicine herbal therapy and survival outcomes in patients with stage II and III colorectal cancer: A multicenter prospective cohort study. J Natl Cancer Inst Monogr. 2017;
2017: lgx015
- Wang Y, Liu P, Fang Y, Tian J, Li S, et al. The effect of long-term traditional Chinese medicine treatment on survival time of colorectal cancer based on propensity score matching: A retrospective
cohort study. Evid Based Complement Alternat Med. 2020; 2020:
7023420.
- Jia R, Liu N, Cai G, Zhang Y, Xiao H, et al. Effect of PRM1201 combined with adjuvant chemotherapy on preventing recurrence and
metastasis of stage III colon cancer: A randomized, double-blind,
placebo-controlled clinical trial. Front Oncol. 2021; 11: 618793.
- En-xin Z. Development of academic connotation of Lingnan School of Traditional Chinese Medicine Oncology by Chinese medical
master ZHOU Dai-han. J Oncol in Chin Med. 2020; 2: 84-88.
- Jingyu Rong LL, Qing Lin, Guanying Qiao. Retrospective study on
traditional Chinese medicine syndrome regularity of colorectal
cancer. J bas and clin oncol. 2015; 28: 238-241.
- Shao C, Zuo Q, Lin J, Yu RJ, Fu Y, et al. Effect of Chinese herbal medicine onthe survival of colorectal cancer patients with liver-limited
666 metastases: A retrospective cohort study, 2008 to 2017. Integr
Cancer Ther. 2019; 18: 1534735419883687.
- Fang R, Wu R, Zuo Q, Yin R, Zhang C, et al. Sophora flavescens
containing-QYJD formula activates Nrf2 anti-oxidant response,
blocks cellular transformation and protects against DSS-induced
colitis in 671 mouse model. Am J Chin Med. 2018; 46: 1609-1623.
- Nakamura Y, Hokuto D, Koyama F. The prognosis and recurrence
pattern of right- and left-Sided colon cancer in stage II, stage III,
and liver metastasis after curative resection. Ann Coloproctol.
2021; 37: 326-336.
- Taieb J, Shi Q, Pederson L, Alberts S, Wolmark N, et al. Prognosis of microsatellite instability and/or mismatch repair deficiency
stage III colon cancer patients after disease recurrence following
adjuvant treatment: results of an ACCENT pooled analysis of seven
studies. Ann Oncol. 2019; 30: 1466-1471
- Muthusami S, Ramachandran IK, Babu KN, Krishnamoorthy S, Guruswamy A, et al. Role of inflammation in the development of colorectal cancer. Endocr Metab Immune Disord Drug Targets. 2021;
21: 77-90.
- Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021; 21: 653-667.
- Tang P, Zha L, Ye C, Zhou L. Research progress on the carcinogenesis mechanism of inflammation in ulcerative colitis: a narrative
review. Ann Palliat Med. 2021; 10: 11994-12002.
- Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer 691 patients. Int J Mol Sci. 2021; 22: 8002.
- Yu X, Wang D, Wang X, Sun S, Zhang Y, et al. CXCL12/CXCR4 promotes inflammation-driven colorectal cancer progression through
694 activation of RhoA signaling by sponging miR-133a-3p. J Exp
695 Clin Cancer Res. 2019; 38: 32.
- Garo LP, Ajay AK, Fujiwara M, Gabriely G, Raheja R, et al. MicroRNA-146a limits tumorigenic inflammation in colorectal cancer. Nat
Commun. 2021; 12: 2419.
- Jackson DN, Theiss AL. Gut bacteria signaling to mitochondria in
intestinal inflammation and cancer. Gut Microbes. 2020; 11: 285-
304.
- Briede I, Balodis D, Gardovskis J, Strumfa I. Stemness, inflammation and epithelial-mesenchymal transition in colorectal Carcinoma: The intricate network. Int J Mol Sci. 2021; 22: 12891.
- Semlali A, Reddy Parine N, Arafah M, Mansour L, Azzi A, et al. Expression and polymorphism of toll-Like receptor 4 and effect on
NF-kappaB mediated inflammation in colon cancer patients. PLoS
One. 2016; 11: e0146333.
- Plewka D, Plewka A, Miskiewicz A, Morek M, Bogunia E. Nuclear
factor-kappa B as potential therapeutic target in human colon cancer. J Cancer Res Ther. 2018; 14: 516-520.
- Sun G, Zheng C, Deng Z, Huang C, Huang J. TRAF5 promotes the
occurrence and development of colon cancer via the activation
of PI3K/AKT/NF-kappaB signaling pathways. J Biol Regul Homeost
Agents. 2020; 34: 1257-1268.
- Huang HY, Zhang ZJ, Cao CB, Wang N, Liu F-F, et al. The TLR4/NF-kappaB signaling pathway mediates the growth of colon cancer.
Eur Rev Med Pharmacol Sci. 2014; 18: 3834-3843