Introduction
Breast cancer is considered one of the common malignancies
in women and an assorted disease at a molecular level. It is considered the 5th common cause of cancer death [1]. Early stages of
breast cancers are treatable and have a higher survival rate, but
coming to advanced stages of breast cancer the survival rate dips
drastically. With the current treatment protocols, the early-stage
breast cancers have almost 100% survival whereas advanced
stage only has a 22% survival [2]. Therefore, there is a pressing
need for understanding the Tumour microenvironment (TME) of
breast cancer for the development of a better treatment protocol
and management of the disease.
A cancer cell simply doesn’t exist in isolation. It forms an environment where it dynamically interacts with other cells and non-cellular components to survive and grow and this is dubbed as
TME. It is composed of blood and lymphatic vessels, fibroblasts,
adipocytes etc acting as stromal cells lymphocytes, macrophages
and other cells, extracellular matrix (ECM) and some secreted
components [3]. In-depth studies have shown how crucial role
TME plays in sustenance, metastatic progression, resistance and
recurrence of the tumour [4,5]. There are studies which show
Mesenchymal stem cells (MSCs) and macrophages as some of
the predominant players of TME in breast cancer. The cross-talk
between these two is being extensively studied and recognized as
one of the pathways that can be used for therapeutic purposes in
breast cancer [6,7].
Immune cells (and inflammation) have long been recognised
as important in regulating and contributing to tumour growth.
Both innate and adaptive immune components actively patrol the
body for incipient tumour cells, and tumor-infiltrating lymphocytes with effector and memory activities have been extensively
characterised inside primary tumours and their metastases. The
existence of an immune infiltration is often linked with a positive
prognosis; however, this is highly dependent on the tumour type,
cell location, and level of activation.
Mesenchymal stem cells (MSCs) have attracted the attention
of the scientific community and extensive work has been carried
out for the past 3 decades for their immunomodulatory properties. MSCs like cells can be obtained from various tissues at all
developmental stages(fetal, young, adult, and aged) but bone
marrow is considered optimal source [8,9].
Immune cells may be seen throughout the TME, and they
contain both innate and adaptive immune cell populations that
interact with tumour tissue-associated MSC, with lymphocytes
accounting for the bulk of tumor-infiltrating immune cells. Monocytes/macrophages, dendritic cells, T cells, B cells, and natural
killer cells all show signs of MSCs’ immunomodulatory capability.
In a complicated interplay initiated by MSCs, anti-inflammatory
monocytes/macrophages and regulatory T cells (Tregs) play a key
role, as they unveil their full immunomodulatory potential.
Macrophages are specialised phagocytic cells in the innate
immune system that play a variety of roles in homeostasis and
immunological responses. Macrophages travel about continually
as scavengers, removing dead cells, harmful bacteria, and other
foreign substances. The activation of macrophages regulates and
reacts to the immune system as a critical modulator and effector cell in the immunological response. The study of MSC-macrophage interactions in tissue homeostasis and injury healing has
gotten a lot of interest in recent years. The control of MSCs on
macrophages will be the focus of this review [10]. The immunomodulatory properties of MSCs were also attributed in breast
cancer progression. The TME was able to subvert MSCs functions
in favour of tumour progression [11].
In TME the immune system is one of the key players in the
cross-talks that happen there. It was observed previously 5-40%
of a solid tumour mass is populated by Tumour-Associated
Macrophages (TAMS) that is associated with a poor prognosis
[12]. Studies have reported that mammary tumours show a paracrine relation between TAMs and cancer cells. Several studies
have shown an association between macrophage infiltration and
angiogenesis in breast cancer. TAMS affect the progression of the
tumour at every step like metastasis, including invasion, vascularization, intravasation, extravasation, establishing pre-metastatic
niches [13,14].
TAMs help establish a microenvironment capable of facilitating
mammary tumour immune evasion through the secretion of soluble factors [15,16]. The cues received from tumour and stromal
cells push the macrophages towards M2 phenotype which are
known for their anti-inflammatory, wound healing properties and
pro-tumour properties. There are several studies which indicate
the increase of TAMs in tumour micro-environment led to poor
clinical outcome and they play a key role in the progression of
angiogenesis, metastasis, immunosuppression and also leading to
chemoresistance [17-19].
Looking at the roles played by MSCs and TAMs in the breast
cancer hints that the crosstalk of MSCs and TAMS might be one
of the major contributors for the sustenance and progression of
TME and breast cancer.
In this review, we want to shed the light on the phenotypic and
functional roles played by the MSCs and TAMs in breast cancer
and the interactions between them aiding the progression of cancer and possible clinical immunotherapies that can be developed
for the breast cancer targeting them.
Mesenchymal stem cells
The works of Friedenstein and his co-workers led to the discovery of mesenchymal stem cells (MSCs), nonhematopoietic
stem cells. The term “mesenchymal stem cells” was proposed by
Caplan in 1991 because of their ability to differentiate into more
than one type of cells that form connective tissue in many organs
[20]. In 2006, The International Society of Cellular Therapy (ISCT)
specified few guidelines which were accepted by the scientific
community and as per the guidelines the use of name multipotent mesenchymal stromal cells was recommended but themesenchymal stem cells (MSCs) remain in use.MSCs like cells can be
obtained from various tissues, but MSCs derived from bone marrow and adipose tissue can create a larger number of CFU-F (colony forming units-fibroblast) colonies, which indirectly indicates
a higher degree of their stemness [21]. The criteria to be met by
cells to be called as MSCs is the growth of the cells in vitro to be
adherent in nature and should express the cluster of differentiation (CD) markers like CD 73, CD 90, CD 105 and should lack the
expression of CD45, CD34, CD14, CD11b, CD79a or CD19. These cells also must possess the ability to differentiate into osteoblasts,
adipocytes, and chondroblasts [22].
One of the key functions of the MSCs is their immunomodulatory properties. It was observed that the MSCs that were grown
in vitro show the ability to interact and regulate the functions of
key effector cells whose major involvement is seen in both innate
and adaptive immunity.
In the past decade, compelling observations were suggesting
certain patterns and pathways that have been constantly repeating which play a key role in MSC-mediated immunomodulation,
operating through a balance in cell contact-dependent mechanisms and soluble factors [23-25]. It was also observed these immunomodulatory features were exploited by solid tumours like
breast cancer. Let’s take a look at these properties in brief.
The major cells of the adaptive arm of the immune system are
T cells which are subdivided into different phenotypes like effector T cells, regulatory T cells (Tregs), and B cells. There are many
studies which observed the modulatory effects of MSCs like suppression of T cell proliferation, inhibiting the differentiation of T
cells into TH1 and TH17 subtypes while enhancing the proliferation of Tregs.
Immunomodulatory effects of MSCs on T cells
T cells are one of the important arsenals of the immune system
against tumours. CD8+ T cells and CD4+ Th1 type T cells help in
combating tumours using means like producing IFN-γ and cytotoxins. In breast cancer, having a high frequency of CD8+ T cells
and CD4+ Th1 type T cells leads to a favourable prognosis [26,27].
However, when higher levels of Th2 and Tregs are seen in breast
Cancer the prognosis is poor and difficult in treating [28,29].
T cells are categorised into various subsets and each plays a
fundamental role in breast cancer progression. The complete picture of their role is still unclear [42]. It has been observed that in
the early stages of breast cancer there is an accumulation of Th17
and Treg cells, with the progression of the tumour the level of
Th17 gradually decreases and the Treg cells increase [43].
It has been shown that MSCs can suppress the T cell proliferation which is induced by the mitogens in vitro. MSCs cause a shift
in T-cell polarization from pro-inflammatory Th1 to anti-inflammatory Th2 cells and also in their secreting cytokine profiles [30-
32]. It has also been observed that MSCs were able to regulate
the production of proinflammatory cytokines like IFN-γ, IL-17 and
TNF-α by TH1 and TH17 cells. It has also been reported that MSCs
were able to boost the production of anti-inflammatory cytokines,
like IL-4 (TH2). These reports the ability of MSCs to favour the
polarization of TH1/TH17 towards TH2 response. MSCs when stimulated by IFN-γ and TNF-α/IL-1 shows their immunosuppressive
potential. MSCs also release indoleamine 2,3-dioxygenase (IDO),
which is enhanced when they are stimulated by IFN-γ. Following
tryptophan deprivation, allogeneic T cell responses are inhibited,
IL-4 secretion in Th2 cells is stimulated, and IFN-γ production in
Th1 cells is decreased.
The MSCs show their suppressive potential on Th1 cytokine
production through PGE2 dependent manner and TH17 through
the up regulation of PD-1 expression and up regulation of IL-10
production. It has also been seen that CCL2 dependent suppression is also used by MSCs for their suppressive functions [33-36].
As we have seen MSCs have immunomodulatory properties which
might be playing a key role here and it has been shown that decrease of TH17 and increase in Tregs leads to poor prognosis and
tough to treat [28,29]. There are also studies showing MSCs promoting the proliferation of regulatory T cells (Tregs). It has been
observed that when MSCs were cocultured with peripheral blood
mononuclear cells (PBMCs) they promote the CD4+ T cells differentiation into Tregs through cell-cell contact-dependent manner.
So, the T-regs induced by the MSC are not expanded from the
existing T-regs, but from the induction of conventional T cells [37-
39].
There are studies which show increase intertumoral infiltration
of CD4+ T cells in breast cancer but it did not bode well with prognosis of the disease [40]. There is also the involvement of soluble
factors like IL-10, TGF-β, IL-6 and PGE2 in this conversion. There
are studies showing that MSCs may directly limit the proliferation
of all reactive CD4+ and CD8+ T cells in the absence of other immune cells, a process mediated in part by MSC-derived galectin-1.
MSCs can decrease T cell activation and promote permanent T
cell hypo-responsiveness and death via secreting PD-L1 [41].
Immunomodulatory effects of MSCs on B cells
Amongst the tumour infiltrating lymphocytes B cells make a
major portion in breast cancer [44]. The diverse functions of B
cells like antigen presentation, cytokine production and interaction with other immune cells enables them to modulate the
immune system pushing the immunity from pro-tumours to anti-tumours. But there are recent reports which show presences of
Bregs in breast cancer which doesn’t give out a favourable prognosis [45].
MSCs suppressive potential has also been seen on B cells. MSCs
were able to inhibit the characteristics of B cells like activation,
differentiation, antibody production, proliferation and chemotaxis. MSCs arrest the proliferation of B cells in the G0/G1 phase
in a paracrine manner. MSCs were able to directly interact with B
cells and can downregulate the plasma-blast formation and promote the development and induction of regulatory B cells (Bregs)
[46]. The Bregs were able to push CD4+ T cells into developing
into Tregs through the production of IL-10. The promotion of Il-10 producing Bregs by MSCs is dependent on cell-to-cell contact
mechanism but not through secretory soluble factor mechanism.
So, for this to happen MSCs should be in a metabolically active
state [47,48].
PD-1 and PDL1 interaction also play a key role in the MSCs suppressive nature on B cells. It has also been observed that MSCs
were able to suppress the conversion of B cells into Plasma cells
by down regulating a master transcription regulator Blimp-1 (B
lymphocyte-induced maturation protein-1) which is required for
the B cell terminal differentiation [49,50]. MSCs were also able to
inhibit the chemotaxis of B-cells by down regulating the expression of receptors like CXCR4, CXCR5, CXCL12 [46].
MSCs interact directly with B cells, reducing plasma blast development and promoting the induction of regulatory B cells
(Bregs). Bregs promote immunological tolerance by its immunosuppressive characteristics. Bregs that produce IL-10 have been
demonstrated to convert Foxp3+ Tregs from effector CD4+ T cells.
MSCs’ stimulatory influence on B-reg formation and IL-10 production appears to be dependent on direct cell-cell contact or
at least close proximity to the relevant cells, rather than soluble
substances. However, it has been demonstrated that MSCs’ stimulatory impact on B-reg creation and inhibitory influence on T
cell proliferation are both dependent on active cell metabolism.
MSC-secreted IL1-RA suppresses B cell development through a
cytokine-triggered mechanism. MSCs reduce B cell growth in the
presence of T cells, which might be owing to IFN-γ secreted by T
cells, as IFN-pre-treated MSCs can also inhibit B cell proliferation.
Infiltrations of Bregs in the solid tumours like breast cancer
has been identified. There are various studies which suggest accumulation of Bregs in the TME might one of the major processes
through which B cells regulate various arms of immunity in the
TME [45]. As the evidence suggests, in the conversion of recruited
B cells into regulatory phenotype MSCs might be playing a major
role which play role in breast cancer progression [48].
Immunomodulatory effects of MSCs on NK cells
The Natural killer cells are one of the major lymphocyte population that plays a key role in the innate arm of immunity. They
exert their cytolytic activity either through Perforin involved pathway or through a caspase-dependent pathway. In the presence
of IL-2 and IL-15, they get activated and MSCs were able to inhibit
the proliferation driven by this mechanism. MSCs were also seen
in interfering with the NK cells ability to produce pro-inflammatory cytokines and cytotoxic molecules like granzyme and perforin.
MSCs also down regulate the expression of activating receptors
like NKp30, NKp44and NKg2D on NK cells [51-53].
Tumor-associated MSCs (T-MSCs) have been found to be one
of the main cells responsible for immunosuppression in the
context of malignancies by producing PGE2. T-MSCs have also
been shown to have a strong inhibitory effect on NK cells by down
regulating the expression of NKG2D, DNAM-I, and NKG2A on NK
cells via direct cell-to-cell contact. Galland et al. (2017) conducted
a comparative research on T-MSCs directly obtained from lung
squamous cell carcinoma tissues, MSCs from neighboring normal
tissues, and BM-MSCs to investigate the suppressive capacities of
these MSCs of various origins. It was discovered that, unlike BM-MSCs, which had a substantial immunosuppressive impact on NK
cells, those obtained from normal tissues had a less effect. While
their findings showed that T-MSCs from tumor-bearing tissues
close to normal sites from which normal MSCs were obtained had
more potent suppressive features than BM-MSCs, they also found
that T-MSCs from tumor-bearing tissues close to normal sites had
more potent suppressive features than BM-MSCs. The inflammatory milieu of the tumour site, which was caused by tumour cells,
stromal cells, and even immune cells, drove the MSCs of the tumour tissues to reclaim powerful BM-MSC-like suppressive capabilities, it was then pointed out [110].
There were recent observations which show the involvement
of NK cells in HER2+ breast cancer [54]. It has been reported the
MSCs were able to exert their immunosuppressive effects on NK
cells by secretion of indoleamine-2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2) [65]. MSCs are also known to secret a soluble
isoform of MSC class II molecule HLA-G5 which tends to interact
with the inhibitory receptors (CD94–NKG2A, KIR2DL4 and ILT-2)
on NK cells and inhibit the NK cells cytolysis functions. These NK cell ligands play an important role in the immune editing of the
tumour and associated with the immune escape of breast cancer. It was observed that upon exposure to IFN-γ MSCs becomes
more resilient towards NK cells by up regulating the MHC class
I expression which inhibits the NK cells function. IFN-γ exposure
also increases the expression of inhibitory proteins like COX2 and
IDO in MHCs [55]. In conditions like breast cancer, the cytotoxic
potential of tumour infiltrating NK cells was substantially impaired compared to the peripheral NK cells which are correlated to
the decrease in expression of activating receptors (NKG2D,.etc)
and increase in expression of inhibitory receptors (NKG2A,.etc)
leading to the survival of cancer [56].
Immunomodulatory effects of MSCs on dendritic cells
Dendritic Cells (DC) act as a bridge between the innate and
adaptive immune systems, through presenting the antigen to
T-cells and playing a key role in regulating their activation and
functions, they also tend to affect B and NK cells by interacting
with them directly [57,58]. Depending upon their activation levels
and subsets Dc’s can either be immune-activating or regulating.
In their immature state Dc’s express no co-stimulatory molecules
and like mature DCs they possess the ability to recognize, process
and present the antigen to T-cells, but in the absence of co-stimulatory receptors on DC’s during interaction push these T-cells towards anergy or apoptosis. Several studies are showing the MSCs
inhibiting the maturation and function of DCs, further inhibiting
the activation and proliferation of T cells. DC’s can directly inhibit
the maturation of monocytes and the precursor cells of DC.
In a tumour microenvironment, MSC have been found to induce monocyte polarisation toward an anti-inflammatory/immune-regulatory (type 2) phenotype and to hinder dendritic cell
development towards the type 1 phenotype (DCs) [115]. MSCs
also prevent DCs from migrating and maturing. DCs are less capable of supporting antigen-specific CD4+ T cell proliferation
and displaying an MHC class II-peptide complex in the presence
of MSCs. When mature type 1 DCs are co-cultured with MSCs,
they produce much less TNF-α, whereas anti-inflammatory mature type 2 DCs secrete significantly more IL-10 [111,116]. Sca-1+CD117L in bone marrow-derived MSCs have also been proven
in mice to create regulatory DCs with immune regulation functions from hematopoietic stem cells [117].
Another question about DC-MSC interactions is whether MSC
can interfere not only with DC formation from precursors, but also
with later phases of differentiation, such as the transition from
immature to mature DC. Several research organisations looked
into this topic and came up with conflicting conclusions. MSC
were shown to moderately decrease LPS-induced monocyte-derived DC maturation in some circumstances. When compared to
control mDC, the resultant cells had a lesser capacity to promote
allogeneic T cell proliferation in MLR, as well as lower levels of IL-12 production and IFN-γ induction.
There are reports in breast cancer patients, DCs have a substantially lower level of expression of HLA-DR and also show lower
expression of MHC-II [59]. The MSCs were able to inhibit DCs at several levels through various regulatory molecules like IL-6, PGE2,
Jagged-2, TSG-6, M-CSF [60-62]. Interaction of DCs with MSCs generates low endocytic capacity, low immunogenicity, and strong
immunoregulatory function DCs. They also have significantly reduced expression of ligands, CD11c, CD80, CD86, and CD40 while
increased expression of CD11b [63] MSCs also secreted Galectin-1
(Gal-1) which upregulated the expression of Gal-1 in DCs which in
turn helped in the formation of tolerance immunophenotype on
DCs, by the regulation of the MAPK signalling pathway [64]. DC’s
seems to be pushed towards tolerogenic subtype in the tumour
environment and MSCs were reported to have a significant role
in it in breast cancer [65]. Such DCs could lead to the induction of
Th2 and Tregs and thereby result in the suppression of pro-inflammatory T cell activation.
Immunomodulatory effects of MSCs on monocytes
MSC have been demonstrated to enhance monocyte/macrophage polarisation toward an anti-inflammatory/immune-regulatory (type 2) phenotype while directly inhibiting development into
the type 1 phenotype [111,112]. Anti-inflammatory monocytes
secrete a lot of IL-10 and have lower levels of IL-12p70, TNF-α,
and IL-17 expression, thanks to MSC-produced IL-6 and hepatocyte growth factor (HGF) [113]. In a positive-feedback loop, monocyte-derived IL-10 limits monocyte development into DCs and
pushes monocytes toward an anti-inflammatory, IL-10-secreting
subtype. MCS-primed monocytes express significant amounts of
MHC class II, CD45R, and CD11b, in addition to IL-10, and appear
to be able to limit T-cell activity in the absence of FoxP3+ Tregs.
CCL-18 generated by monocytes and transforming growth factor
beta 1 (TGFβ-1) secreted by monocytes are both involved in monocyte-induced Treg development [114].
Immunomodulatory effects of MSCs on macrophages
Macrophages (Mφ) are one of the innate immune system cells
that are known for their phagocytic activity and immune properties. They are generally categorized into two phenotypes, the
M1 macrophages a pro-inflammatory phenotype and M2 macrophages an anti-inflammatory or regulatory phenotype. They are
categorized depending on their secretory profile and the surface
receptor expression. Studies were showing the MSCs working in
symbiosis with macrophages to maintain tissue homeostasis.
Mφ have a lengthy life lifetime in diverse tissues, with some
surviving for months to years. Despite the fact that Mφ have the
ability to proliferate in tissues, they seldom divide and are mostly
replaced by monocyte migration in the blood. Mφ can be separated into two types based on distinct activation methods: conventionally activated Mφ (M1) and alternatively activated Mφ (M2).
Surface receptor expression, cytokine and chemokine production,
effector functions, and so on differ between the two kinds.
M1-type cells can be activated by cytokines such as interferon gamma (IFN-γ), lipopolysaccharide (LPS), granulocyte-macrophage colony stimulating factor (GM-CSF), or tumour necrosis
factor (TNF), which results in increased self-antigen presentation,
complement-mediated phagocytic activity, proinflammatory factor release (IL-1, TNF-, IL-12, IL-6, IL (CXCL9, CXCL10, etc.) M1
cells can stimulate the removal of non-self-components in vivo
and play an essential role in tumour prevention by releasing these
inflammatory mediators. M1 cells also act as effector cells in the
Th1-mediated immune response, increasing inflammation and killing intracellular pathogens.
M2a, M2b, and M2c cells are subtypes of M2-type cells. M2a
is triggered by IL-13 or IL-4, whereas M2b is triggered by Toll-like receptor (TLR) ligands and IL-10. M2a and M2b cells primarily regulate the immune system by encouraging the Th2-mediated immune response. M2c cells’ major job is to prevent the occurrence
of the immune response, which is crucial in the process of tissue
remodeling.
In an inflammatory environment when the monocytes enter,
they respond to the local stimuli and either differentiate into M1
macrophages secreting cytokines like IFN-γ and TNF-α and add to
the inflammation or they might develop into M2 macrophages
and secret IL-10 and TGF-β trying to douse the inflammation. The
transition of monocytes to M1 or M2 is influenced by many factors and the threshold of type of signal they receive also influence
them in this transition.
The macrophages, which are influenced by MSCs likely to have
an increased proliferative and migratory potential [66]. Macrophages influenced by MSCs have a great potential in impairing T-cell response and inducing T-regs. MSCs under the action of IFN-γ
and TNF-α in an inflammatory setup gains a greater ability to influence the shift of macrophages from M1 to M2 by expressing
higher concentrations of COX2 and IDO and also induces T-regs at
a greater capacity and inhibit effector T cell response [67,68]. Certain pathways had been identified through which MSCs influence
macrophage functions. It was observed that MSCs released factors like TNF-stimulated gene 6 (TSG6) which effected the TLR2–nuclear factor-κB (NF-κB) signalling which hindered the activation
of peripheral macrophages [69].
In breast cancer tumour micro-environment setup, MSCs were
observed to release a plethora of cytokines like CCL-2, CCL-7 and
CCL-12 due to which there is increased recruitment of CCR2 expressing monocytes and macrophages which aid in the tumour
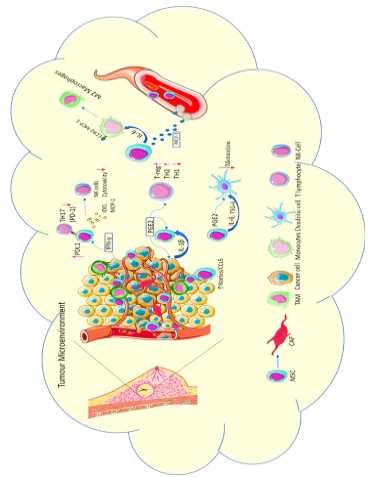
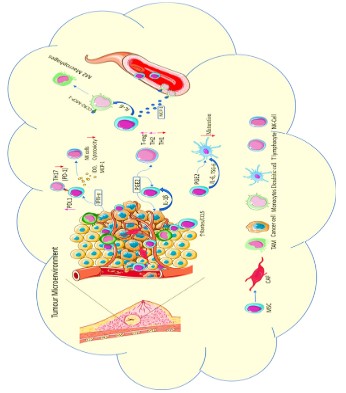
progression [70] (Figure 1).
The MSCs are found in different parts of the body which were
able to migrate all over the body as well as into the tumour [71].
Due to the chronic inflammatory conditions, neovascularization
and infiltration of immune cells tumours are considered “wound
which doesn’t heal” it enables TME to recruit MSCs as well.
Though bone marrow is considered a major source the MSCs also
spring from the adipose tissue surrounding it [72].
The MSCs in the TME undergo differentiation into CAFs and
express FAP (Fibroblast activation protein) and FSP (Fibroblast
specific protein) whereas the FAP is expression is generally not
detectable in healthy tissues [73-75].
In a tumour microenvironment, the immune system is one of
the key players in the cross-talks that happen there. It was observed previously 5-40% of a solid tumour mass is populated by
tumour-associated macrophages (TAMS) that is associated with a
poor prognosis [12,14]. TAMS are the macrophages that were recruited and educated in the tumour microenvironment. They are
exposed to regulatory cytokines like IL-10 and TGF-β making them
M2 like phenotype [76,77]. Thanks to their M2 like phenotype
TAMS were observed to carry out immunosuppressive function
rather than immune effector [14].
The dominant phenotype of TAMS in breast cancer is M2 phenotype which is known for their tumour promoting [78]. TAMs
are known to promote tumour growth, invasion, angiogenesis
[79,80]. Studies have reported that mammary tumours show a
paracrine relation between TAMs and cancer cells. Monocyte colony-stimulating factor receptor (M-CSFR, also known as CSF-1R
or cFMS) is expressed by TAMS, which binds to monocyte colony-stimulating factor (M-CSF, also known as CSF-1) secreted by
cancer cells. Likewise, Epidermal Growth Factor (EGF) secreted by
TAMS and activate the EGF receptor (EGFR) on the cancer cells.
This facilitates the co-migration of the two cell types, which enhances the motility and subsequent invasion into surrounding
healthy tissue [15,81].
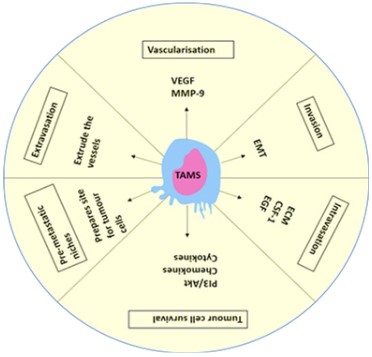
Several studies have shown an association between macrophage infiltration and angiogenesis in breast cancer. TAMS affect
the progression of the tumour at every step like metastasis, including invasion, vascularization, intravasation, extravasation, establishing pre-metastatic niches [13,16,82,83]. TAMs expressing
the macrophage Colony-Stimulating Factor (CSF1) and its receptor
(CSF1R) correlated to poor prognosis in breast cancer [84].
Macrophages present in mammary tumours undergo a profound reduction of MHC class II expression mediated by tumour-derived Migration Inhibitory Factor (MIF), inhibiting subsequent
antigen presentation and adaptive immune induction. Owing to
their abundance within mammary tumours, the loss of tumoricidal function by macrophages represents a crucial breach in immunosurveillance required for breast cancer development and
progression [85] (Figure 2).
MSCs role in Cancer and crosstalk with TAMS
In recent decades in-depth studies have been conducted on
the relation between MSCs and Cancer. But there isn’t any conclusive answer to this question. There are studies showing cancer
progression and metastasis promoting actions by MSCs [86,87].
There are also studies showing the suppressive effect of MSCs
on the proliferation of Tumour. Studies had shown recruitment of MSCs to the tumour sites which promoted the growth of the
tumour. The transformation of MSCs into cancer-associated myofibroblasts was also observed which secret the angiogenic cytokines like IL-6, VEGF and TGF-β [88-90]. These recruited MSCs promote metastasis by lysyl oxidase up regulation. The immunomodulatory properties of MSCs were also attributed in breast cancer
progression. The MSCs modulation of Tregs and down regulation
of NK cells and Cytotoxic T Lymphocytes (CTL) functions helped
in breast cancer progression [91,92]. There is the recruitment of
MSCs to the tumour microenvironment where they differentiate
into Cancer-Associated Fibroblasts (CAFs) which promotes the tumour progression.
MSCs secrete an array of molecules depending on the environmental cues it gets. In tumour microenvironment also they secrete
several molecules which play an important role in the tumour’s
fate. In ovarian cancer, MSCs were found to secrete (Bone morphogenetic Proteins) BMP2 and 4 which increases the number of
cancer stem cells (CSCs) [93]. In-turn this CSCs activate and up regulate the Hedgehog pathway. In breast cancer, MSCs were found
to induce and up regulate the expression of mir-199 and mir214
leading to the down regulation of FoxP2 promoting survivability if
CSCs and progression of metastasis [94]. The cancer cells release
IL-1α and IL-1β which induces the expression of PGE2, IL-6 and IL-8
by MSCs leading to the production of CXCL1 and CXCL8 promoting
the stemness characteristics in the tumour microenvironment.
MSCs were able to propagate the breast cancer cells positive
for Aldehyde Dehydrogenase (ALDH) through the production of
CXCR2 which induces the expression of Sox2 and Oct4 [95,96].
MSCs also secrete IL-6 which up regulates the CD133 expression
through the JAK2-STAT3 pathway in CSCs. Studies are showing
when MSCs were cocultured with breast cancer cells, promoted the expression of CXCR2 ligands including CXCL 1,5,6,7 and
8 which supported the developments of CSCs. There is also an
increase in breast cancer CSCs when MSCs secrete cytokines like
IL-10 IL-17b and proteins like EGF. MSCs were also seen regulating the metabolism of CSCs through exosome production in breast
cancer. CCL5 secreted by MSCs promotes the growth of breast
cancer and its invasive properties [97,98]. In the progression of
tumour macrophages are one of the immune cells that demands
due attention. The macrophages and monocytes are recruited to
the tumour microenvironment which alters and accelerate the tumour progression. The cues received from tumour and stromal
cells push the macrophages towards M2 phenotype which are
known for their anti-inflammatory, wound healing properties and
pro-tumour properties. These M2- polarized macrophages closely
resemble the Tumour-associated Macrophages (TAMs) that are
important for the tumour microenvironment. There are several
studies which indicate the increase of TAMs in tumour micro-environment led to poor clinical outcome and they play a key role in
the progression of angiogenesis, metastasis, immunosuppression
and also leading to chemoresistance [17-19].
The metastasis initiation requires the invasion which is triggered by Epithelial-Mesenchymal Transition (EMT) pathway. This is
a process in which the epithelial cell loses its cell-to-cell adhesion
and cell polarity and gain migratory and invasive properties. The
MSCs present in the tumour microenvironment may stimulate the
EMT pathway of tumour cells. Studies are showing breast cancer
cells, when co-cultured with human bone marrow, derived MSCs
showed increased EMT markers (N-cadherin, vimentin, Twist and
Snail) and decrease of E-cadherin [99,100].
In breast cancer it was observed, adipose-derived MSCs induce an upregulation of EMT related genes. The method of action
through which MSCs exerts its effect on the tumour is not yet fully
elucidated but there are studies which reported when the MSCs
were cocultured along with breast cancer cells, they promoted
the elongation, directional migration and traction of cancer cells.
All this was possible through MSCs secreted TGF-β, focal adhesion
kinases, matrix metalloproteases and migratory proteins [101]
(Figure 3).
In tumour microenvironment the MSCs secret a varied series
of growth factors, cytokines and chemokines which are known to
influence the breast cancer TME and help in tumour progression,
migration and angiogenesis [102].
The impact of cytokines on metastasis, angiogenesis and tumour progression. The type and levels of cytokines vary amongst
various stages of breast cancer i.e., from early to metastatic [103].
The breast cancer TME exhibit an inflamed cytokine profile leading to a poor clinical outcome [104,105]. Upon the progression
of breast cancer progression to advance stages the expression of
TGF-βis increased which helps in the tumour stemness, immune
suppression and treatment resistance [106]. Reports are showing
the production of TGF-β by MSCs which play one of the key roles
in the metastasis of breast cancer [101] and also enhances the
EMT progression [91]. In the breast cancer upon the interaction
of IFN-γ and TNF-α, MSCs tends to produce TGF-β promoting EMT,
migration and invasion of breast cancer [107]. Breast cancer cells
also secret high levels of IL-6 which also attracts and activates the
MSC under the hypoxia conditions [108]. The MSCs also tend to
secret IL-6 in breast cancer TME in hypoxia condition and promotes tumour immune evasion and polarization of macrophages
to M2 phenotype [107]. IL-6 by MSCs also stimulates STAT-3 phosphorylation and promotes breast cancer cell progression and migration the tumour growth and metastasis is also promoted by
MSCs by secreting enzyme matrix metalloproteinase16 (MMP16)
[99]. MSCs were able to modulate the stemness of the breast cancer cells through cytokine production like IL-6 and chemokine ligands -7 (CXCL-7) [109].
Conclusion
The role of MSCs has been implicated in several stages of the
tumour progression. Due to the secretion factor secreted by the
cancer cells recruit the MSCs to cancer microenvironment. In the
TME the MSCs are tend to differentiate into CAFs which help in
the tumour progression. In the breast cancer environment, MSCs
promote the EMT of cancer cells which play a key role in the cancer progression. MSCs also promote breast cancer metastasis.
MSCs through their immunomodulatory properties had been
seen to affect the immune cells that invade the breast cancer TME
and promote the cancer immune escape, treatment resistance.
Through paracrine and direct contact action the MSCs were able
to promote the stemness and angiogenesis. Even though studies
are showing the MSCs anti-tumour affect the overall net effect
seems to be tumour progression.
In this review we have discussed MSCs and the mechanisms
through which they exert their immunomodulatory effect on both
adaptive and innate immunity in the cancer microenvironment,
emphasising both cells to cell interaction and paracrine effect. We have also discussed the role of TAMs in TME and how they help in
breast cancer progression and the cross-talk between MSCs and
TAMs. Having a better understanding of immunomodulatory interaction MSCs with lymphocytes and TAMs and their cross-talk
with breast cancer stroma will enable in designing a better treatment modality for combating breast cancer.
Declarations
Conflict of interest: The authors declare that no competing financial interest.
Acknowledgements: We would like to extend my thanks to Dr
Kunal for helping in getting this review into final shape by providing their valuable suggestions and inputs.
Funding: The authors sincerely express their utmost gratitude
to the Council of Scientific and Industrial Research (CSIR) for research grant funding (No-09/006(0469)/2017-EMR-I) and All India
Institute of Medical Sciences, New Delhi, Indiaand DST-Serb project (DST/INSPIRE/04/2016/001027), India for giving the facilities
for completion of the research work.
Ethical approval: This article does not contain any studies with
human participants performed by any of the authors.
References
- Assessing national capacity for the prevention and control of non-communicable diseases: report of the 2019 global survey. Geneva:
World Health Organization; 2020.
- Alkabban FM, Ferguson T. Breast Cancer. In: Stat Pearls. Treasure
Island (FL): Stat Pearls Publishing; 2022
- Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting Tumor Microenvironment for Cancer Therapy. Int J Mol Sci.
2019; 20: 840.
- Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, et al. Extracellular
vesicles-mediated intercellular communication: roles in the tumor
microenvironment and anti-cancer drug resistance. Mol Cancer.
2019; 18:55.
- Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately
Modulates Cancer Progression. Cancer Res. 2019; 79: 4557-4566.
- Biswas S, Mandal G, Chowdhury SR, Purohit S, Payne KK, et al.
Mesenchymal stem cells educate breast tumor associated macrophages to acquire increased immunosuppressive features. The
Journal of Immunology. 2019; 202: 135.125.
- Weber CE, Kothari AN, Wai PY, Li NY, Driver J, et al. Osteopontin
mediates an MZF1-TGF-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene. 2015; 34: 4821-4833.
- Hass R, Kasper C, Böhm S, Jacobs R. Different populations and
sources of human mesenchymal stem cells (MSC): A comparison
of adult and neonatal tissue-derived MSC. Cell Commun Signal.
2011; 9: 12.
- Elahi KC, Klein G, Avci-Adali M, Sievert KD, MacNeil S, et al. Human Mesenchymal Stromal Cells from Different Sources Diverge in
Their Expression of Cell Surface Proteins and Display Distinct Differentiation Patterns. Stem Cells Int. 2016; 2016: 5646384.
- Jiang Y, Wells A, Sylakowski K, Clark AM, Ma B. Adult Stem Cell
Functioning in the Tumor Micro-Environment. Int J Mol Sci. 2019; 20: 2566.
- Galland S, Stamenkovic I. Mesenchymal stromal cells in cancer: a
review of their immunomodulatory functions and dual effects on
tumor progression. J Pathol. 2020; 250: 555-572.
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated
macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002; 196: 254-265.
- Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, et al. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012; 65: 159-163.
- Pollard JW. Macrophages define the invasive microenvironment in
breast cancer. J Leukoc Biol. 2008; 84: 623-630.
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, et al. A paracrine loop
between tumor cells and macrophages is required for tumor cell
migration in mammary tumors. Cancer Res. 2004; 64: 7022-7029.
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, et al. Association of macrophage infiltration with angiogenesis and prognosis
in invasive breast carcinoma. Cancer Res. 1996; 56: 4625-4629.
- Mantovani A. MSCs, macrophages, and cancer: a dangerous ménage-à-trois. Cell Stem Cell. 2012; 11: 730-732.
- Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers
(Basel). 2014; 6: 1670-1690.
- Jia XH, Feng GW, Wang ZL, Du Y, Shen C, et al. Activation of mesenchymal stem cells by macrophages promotes tumor progression
through immune suppressive effects. Oncotarget. 2016; 7: 20934-
20944.
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991; 9: 641-
650.
- Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal
stromal cells for therapy. Nat Biomed Eng. 2019; 3: 90-104.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et
al. Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315-317.
- Wu Y, Hoogduijn MJ, Baan CC, Korevaar SS, de Kuiper R, et al. Adipose Tissue-Derived Mesenchymal Stem Cells Have a Heterogenic
Cytokine Secretion Profile. Stem Cells Int. 2017; 2017: 4960831.
- de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A,
et al. Immunomodulation By Therapeutic Mesenchymal Stromal
Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells. 2018; 36: 602-615.
- Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, et al.
Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal
stem cell-supported minimized immunosuppressive therapy. J Immunol. 2014; 193: 4988-4999.
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, et
al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in
breast cancer. J Clin Oncol. 2011; 29: 1949-1955.
- Fang D, Zhu J. Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate
lymphoid cell subsets. J Exp Med. 2017; 214: 1861-1876.
- Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by
promoting type 2 inflammation. J Exp Med. 2011; 208: 479-490.
- Wang L, Simons DL, Lu X, Tu TY, Solomon S, et al. Connecting blood
and intratumoral Treg cell activity in predicting future relapse in
breast cancer. Nat Immunol. 2019; 20: 1220-1230.
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, et al.
Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood.
2002; 99: 3838-3843.
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal
cells: implications in transplantation. Transplantation. 2003; 75:
389-397.
- Wang Q, Sun B, Wang D, Ji Y, Kong Q, et al. Murine bone marrow
mesenchymal stem cells cause mature dendritic cells to promote
T-cell tolerance. Scand J Immunol. 2008; 68: 607-615.
- Darlington PJ, Boivin MN, Renoux C, François M, Galipeau J, et al.
Reciprocal Th1 and Th17 regulation by mesenchymal stem cells:
Implication for multiple sclerosis. Ann Neurol. 2010; 68: 540-545.
- Mohammadzadeh A, Pourfathollah AA, Shahrokhi S, Hashemi SM,
Moradi SL, et al. Immunomodulatory effects of adipose-derived
mesenchymal stem cells on the gene expression of major transcription factors of T cell subsets. Int Immunopharmacol. 2014; 20:
316-321.
- Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, et
al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011; 41: 2840-2851.
- Luz-Crawford P, Noël D, Fernandez X, Khoury M, Figueroa F, et al.
Mesenchymal stem cells repress Th17 molecular program through
the PD-1 pathway. PLoS One. 2012; 7: e45272.
- Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and
switchers of inflammation. Cell Stem Cell. 2013; 13: 392-402.
- Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation
of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007; 92: 881-888.
- Engela AU, Hoogduijn MJ, Boer K, Litjens N H R , Betjes M G H , et
al. Human adipose-tissue derived mesenchymal stem cells induce
functional de-novo regulatory T cells with methylated FOXP3 gene
DNA. Clin Exp Immunol. 2013; 173: 343-354.
- Deshmukh AV, Gupta A, Rathod RR, Gangane NM, et al. Role of
CD4- and CD8-Positive T Cells in Breast Cancer Progression and
Outcome: A Pilot Study of 47 Cases in Central India Region. Indian
J Gynecol Oncolog. 2020; 18: 109.
- English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, et al. Cell contact,
prostaglandin E(2) and transforming growth factor beta 1 play
non-redundant roles in human mesenchymal stem cell induction
of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp
Immunol. 2009; 156: 149-160.
- Speiser DE, Verdeil G. More T Cells versus Better T Cells in Patients
with Breast Cancer. Cancer Discov. 2017; 7: 1062-1064.
- Wang J, Cai D, Ma B, Wu G, Wu J. Skewing the balance of regulatory T-cells and T-helper 17 cells in breast cancer patients. J Int
Med Res. 2011; 39: 691-701
- Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary
observations. J Clin Pathol. 2006; 59: 972-977.
- Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer.
2016; 4: 40.
- Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal
stem cells modulate B-cell functions. Blood. 2006; 107: 367-372.
- Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A,
et al. Mice lacking endogenous IL-10-producing regulatory B cells
develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011; 186: 5569-5579.
- Luk F, Carreras-Planella L, Korevaar SS, de Witte SFH, Borràs FE,
et al. Inflammatory Conditions Dictate the Effect of Mesenchymal
Stem or Stromal Cells on B Cell Function. Front Immunol. 2017;
8:1042.
- Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal
progenitor cells inhibit lymphocyte proliferation by activation of
the programmed death 1 pathway. Eur J Immunol. 2005; 35:1482-1490.
- Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, et al. Mesenchymal
stem cells suppress B-cell terminal differentiation. Exp Hematol.
2009; 37: 604-615.
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and
natural killer cells. Stem Cells. 2006; 24: 74-85.
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L.
Mesenchymal stem cell-natural killer cell interactions: evidence
that activated NK cells are capable of killing MSCs, whereas MSCs
can inhibit IL-2-induced NK-cell proliferation. Blood. 2006; 107:
1484-1490.
- Poggi A, Prevosto C, Massaro AM, Negrini S, Urbani S, et al. Interaction between human NK cells and bone marrow stromal cells
induces NK cell triggering: role of NKp30 and NKG2D receptors. J
Immunol. 2005; 175: 6352-6360.
- Muntasell A, Rojo F, Servitja S, Rubio-Perez C, Cabo M, et al. NK
Cell Infiltrates and HLA Class I Expression in Primary HER2+ Breast
Cancer Predict and Uncouple Pathological Response and Disease-free Survival. Clin Cancer Res. 2019; 25: 1535-1545
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari
MC, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine
2,3-dioxygenase and prostaglandin E2. Blood. 2008; 111: 1327-
1333.
- Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier
J, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest.
2011; 121: 3609-3622.
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, et al.
Reciprocal activating interaction between natural killer cells and
dendritic cells. J Exp Med. 2002; 195: 327-333.
- Dubois B, Bridon JM, Fayette J, Barthélémy C,Banchereau J, et al.
Dendritic cells directly modulate B cell growth and differentiation.
J Leukoc Biol. 1999; 66: 224-230.
- Gervais A, Levêque J, Bouet-Toussaint F, Burtin F, Lesimple T, et al. Dendritic cells are defective in breast cancer patients: a potential
role for polyamine in this immunodeficiency. Breast Cancer Res.
2005; 7: R326-R335.
- Zhang B, Liu R, Shi D, Liu X, Chen Y, et al. Mesenchymal stem cells
induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009; 113: 46-57.
- Liu Y, Yin Z, Zhang R, Yan K, Chen L, et al. MSCs inhibit bone marrow-derived DC maturation and function through the release of
TSG-6. Biochem Biophys Res Commun. 2014; 450: 1409-1415.
- Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE.
Mesenchymal stem cells inhibit generation and function of both
CD34+-derived and monocyte-derived dendritic cells. J Immunol.
2006; 177: 2080-2087.
- Zhang Y, Ge XH, Guo XJ, Guan SB, Li XM,et al. Bone Marrow Mesenchymal Stem Cells Inhibit the Function of Dendritic Cells by Secreting Galectin-1. Biomed Res Int. 2017; 2017: 3248605.
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005; 105: 2214-
2219
- Chen X, Shao Q, Hao S, Zhao Z, Wang Y, et al. CTLA-4 positive breast
cancer cells suppress dendritic cells maturation and function. Oncotarget. 2017; 8: 13703-13715
- Gao S, Mao F, Zhang B, Zhang L, Zhang X, et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and
activator of transcription 3 pathways. Exp Biol Med (Maywood).
2014; 239: 366-375.
- Kim J, Hematti P. Mesenchymal stem cell-educated macrophages:
a novel type of alternatively activated macrophages. Exp Hematol.
2009; 37: 1445-1453.
- Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn
MJ, et al. Multipotent stromal cells induce human regulatory T
cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013; 31: 1980-
1991.
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory
protein TSG-6 secreted by activated MSCs attenuates zymosaninduced mouse peritonitis by decreasing TLR2/NF-κB signaling in
resident macrophages. Blood. 2011; 118: 330-338.
- Ren G, Zhao X, Wang Y, Zhang X, Chen X, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα.
Cell Stem Cell. 2012; 11: 812-824.
- Blonska M, Agarwal NK, Vega F. Shaping of the tumor microenvironment: Stromal cells and vessels. Semin Cancer Biol. 2015; 34:
3-13.
- Dvorak HF. Tumors: wounds that do not heal. Similarities between
tumor stroma generation and wound healing. N Engl J Med. 1986;
315: 1650-1659.
- Grisendi G, Spano C, Rossignoli F, Souza ND, Golinelli G, et al. Tumor Stroma Manipulation By MSC. Curr Drug Targets. 2016; 17:
1111-1126
- Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR. Targeting carcinoma-associated fibroblasts within the tumor stroma
with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst. 2012; 104: 1320-1334.
- Guan J, Chen J. Mesenchymal stem cells in the tumor microenvironment. Biomed Rep. 2013; 1: 517-521.
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage
polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002; 23:
549-555.
- Grugan KD, McCabe FL, Kinder M, Greenplate AR, Harman BC, et
al. Tumor-associated macrophages promote invasion while retaining Fc-dependent anti-tumor function. J Immunol. 2012; 189:
5457-5466.
- Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017; 5: 53.
- Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, et
al. Tumor-associated macrophages in breast cancer: Innocent bystander or important player?. Cancer Treat Rev. 2018; 70: 178-189.
- Xu M, Liu M, Du X, Li S, Li H, et al. Intratumoral Delivery of IL-21
Overcomes Anti-Her2/Neu Resistance through Shifting Tumor-Associated Macrophages from M2 to M1 Phenotype. J Immunol.
2015; 194: 4997-5006.
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011; 475: 222-225.
- Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression
analysis of macrophages that facilitate tumor invasion supports a
role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010; 184: 702-712
- Bergenfelz C, Medrek C, Ekström E, Jirström K, Janols H et al. Wn-t5a induces a tolerogenic phenotype of macrophages in sepsis and
breast cancer patients. J Immunol. 2012; 188: 5448-5458.
- Richardsen E, Uglehus RD, Johnsen SH, Busund LT. Macrophagecolony stimulating factor (CSF1) predicts breast cancer progression
and mortality. Anticancer Res. 2015; 35: 865-874.
- Zhang M, Yan L, Kim JA. Modulating mammary tumor growth, metastasis and immunosuppression by siRNA-induced MIF reduction in tumor microenvironment. Cancer Gene Ther. 2015; 22: 463-474.
- Zhong W, Tong Y, Li Y, Yuan J, Hu S, et al. Mesenchymal stem cells
in inflammatory microenvironment potently promote metastatic
growth of cholangiocarcinoma via activating Akt/NF-κB signaling
by paracrine CCL5. Oncotarget. 2017; 8: 73693-73704.
- Wang W, Zhong W, Yuan J, Yan C, Hu S, et al. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget. 2015; 6: 42276-42289
- Tsai KS, Yang SH, Lei YP, Tsai CC, Chen HS, et al. Mesenchymal stem
cells promote formation of colorectal tumors in mice. Gastroenterology. 2011; 141: 1046-1056.
- Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of
breast cancer cells. Oncogene. 2009; 28: 2745-2755.
- Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, et al. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis
of breast and prostate tumors. Stem Cell Res Ther. 2013; 4: 70.
- El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, et al. Critical
role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci U S A. 2012; 109: 17460-17465.
- Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar
P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stemcell-derivedTGF-beta. J
Immunol. 2010; 184: 5885-5894.
- McLean K, Gong Y, Choi Y, Deng N, Yang K, et al. Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem
cells and tumorigenesis via altered BMP production. J Clin Invest.
2011; 121: 3206-3219.
- Coffman LG, Choi YJ, McLean K, Allen BL, di Magliano MP, et al.
Human carcinoma-associated mesenchymal stem cells promote
ovarian cancer chemotherapy resistance via a BMP4/HH signaling
loop. Oncotarget. 2016; 7: 6916-6932.
- Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and
promote breast cancer metastasis. Cell Stem Cell. 2014; 15: 762-
774.
- Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via
prostaglandin E2 signaling. Cancer Discov. 2012; 2: 840-855.
- Pinilla S, Alt E, Abdul Khalek FJ, Jotzu C, Muehlberg F, et al. Tissue
resident stem cells produce CCL5 under the influence of cancer
cells and thereby promote breast cancer cell invasion. Cancer Lett.
2009; 284: 80-85.
- Lazennec G, Lam PY. Recent discoveries concerning the tumor-mesenchymal stem cell interactions. Biochim Biophys Acta. 2016;
1866: 290-299.
- Xue Z, Wu X, Chen X, Liu Y, Wang X, et al. Mesenchymal stem cells
promote epithelial to mesenchymal transition and metastasis in
gastric cancer though paracrine cues and close physical contact. J
Cell Biochem. 2015; 116: 618-627.
- Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, et al. Potential
role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat. 2010; 124: 317-326.
- McAndrews KM, McGrail DJ, Ravikumar N, Dawson MR. Mesenchymal Stem Cells Induce Directional Migration of Invasive Breast
Cancer Cells through TGF-β. Sci Rep. 2015; 5: 16941.
- Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F. Concise review: Dissecting a discrepancy in the literature: do mesenchymal
stem cells support or suppress tumor growth?. Stem Cells. 2011;
29: 11-19.
- Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the Treatment of Cancer. J Interferon Cytokine Res. 2019; 39: 6-21.
- Wu TC, Xu K, Martinek J, Young RR, Banchereau R, et al. IL1 Receptor Antagonist Controls Transcriptional Signature of Inflammation
in Patients with Metastatic Breast Cancer. Cancer Res. 2018; 78:
5243-5258.
- Kawaguchi K, Sakurai M, Yamamoto Y, Suzuki E, Tsuda M, et al. Alteration of specific cytokine expression patterns in patients with
breast cancer. Sci Rep. 2019; 9: 2924.
- David CJ, Massagué J. Contextual determinants of TGFβ action in
development, immunity and cancer [published correction appears
in Nat Rev Mol Cell Biol. Nat Rev Mol Cell Biol. 2018; 19: 419-435.
- Zhang J, Cao J, Ma S, Dong R, Meng W, et al. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK
signaling. Oncotarget. 2014; 5: 9664-9677
- Rattigan Y, Hsu JM, Mishra PJ, Glod J, Banerjee D. Interleukin 6
mediated recruitment of mesenchymal stem cells to the hypoxic
tumor milieu. Exp Cell Res. 2010; 316: 3417-3424.
- Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine. Cancer Res.
2011; 71: 614-624
- Moloudizargari M, Govahi A, Fallah M, Rezvanfar MA, Asghari MH,
et al. The mechanisms of cellular crosstalk between mesenchymal
stem cells and natural killer cells: Therapeutic implications. J Cell
Physiol. 2021; 236: 2413-2429.
- Zheng G, Huang R, Qiu G, Ge M, Wang J, et al. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue
Res. 2018; 374: 1-15.
- Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn
MJ, et al. Multipotent stromal cells induce human regulatory T
cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013; 31: 1980-19891.
- Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica. 2013; 98: 888-895
- Chang Y, de Nadai P, Azzaoui I, Morales O, Delhem N, et al. The chemokine CCL18 generates adaptive regulatory T cells from memory
CD4+ T cells of healthy but not allergic subjects. FASEB J. 2010;
24:5063-5072
- Deng Y, Zhang Y, Ye L, Zhang T, Cheng J, Chen G, et al. Umbilical
cord-derived mesenchymal stem cells instruct monocytes towards
an IL10-producing phenotype by secreting IL6 and HGF. Sci Rep.
2016; 6: 37566.
- Azzaoui I, Yahia SA, Chang Y, Vorng H, Morales O, Fan Y, et al. CCL18
differentiates dendritic cells in tolerogenic cells able to prime regulatory T cells in healthy subjects. Blood. 2011; 118: 3549-3558.
- Liu X, Ren S, Ge C, Cheng K, Zenke M, et al. Sca-1+Lin-CD117- mesenchymal stem/stromal cells induce the generation of novel IRF8-controlled regulatory dendritic cells through Notch-RBP-J signaling. J Immunol. 2015; 194:4298-4308.