Introduction
Cervical cancer is a common gynecological malignant tumor in
clinical practice. As the second most common cancer among femal in the world, cervical cancer has become the second leading
cause of death of malignant tumors in the female genital system
and posing a serious threat to the safety and health of female in
China [1]. Cervical cancer is a long-term process and it takes a
long time (5 to 10 years) to develop from cervical of precancerous
lesions to Cervical Intraepithelial Neoplasia (CIN). Therefore, early
diagnosis and treatment of cervical cancer patients is of great significance to improve the prognosis of patients [2]. Patients with
early cervical cancer have no conscious symptoms, and their cervical tissues are also lack of special changes with naked-eye, leading to missed diagnosis or misdiagnosis in clinical examination
that affects early treatment of patients [3]. Therefore, it is very
important to select reasonable and effective detection methods
to improve the early diagnosis rate of cervical cancer. The studies found that the expression of SMARCE1 in cancer tissues of
patients with gastric cancer, ovarian carcinoma and liver cancer
are closely related to prognosis, and SMARCE1 is a critical gene to
promote the invasion and metastasis of breast carcinoma cells [4-6]. Cysteine-Rich Secretory Protein 3 (CRISP3) is the third member
of the cysteine-rich secretory protein family that has been confirmed to be low expressed in carcinoma of prostate, breast carcinoma and ovarian carcinoma, and the low expression of CRISP3
is related to the sur the stimulation reaction of malignant tumor
cells or the body by tumors, the levels avival rate of breast carcinoma patients [7]. In addition, tumor markers refer to the special
biochemical substances that exist in the body fluid, urine or blood
of tumor patients and are generated byre higher than those of
normal people, the changes can reflect the occurrence and development of tumors and play a role in early screening of cancer
[8]. The study is to explore the diagnostic value of SMARCE1 and
CRISP3 combined with tumor markers in cervical cancer, so as to
provide reference for clinical diagnosis and treatment.
Materials and methods
General materials
80 patients with cervical diseases were diagnosed and treated
in Tianjin Fifth Central Hospital from January 2020 to March 2022,
and were divided into the control group (with chronic cervicitis,
n=30) and the observation group (with cervical cancer, n=50) according to the pathological examination results. The observation
group was 35~58 years old, with an average age of (47.63 ± 2.75)
years. The control group was 35~57 years old, with an average
age of (47.34 ± 3.12) years. There was no significant difference in
age between the two groups (P>0.05). The study was reviewed
and approved by the Ethics Committee of the Tianjin Fifth Central Hospital, and all subjects signed the informed consent form.
Inclusive criteria: I. Patients diagnosed as chronic cervicitis or
cervical cancer by pathological examination [9]. II. No previous
pelvic radiation history. III. Patients with complete clinical medical records. Exclusion criteria: I. Patients have received drug and
surgical treatment for cervical diseases. II. Patients with other
known tumors. III. Patients with severe heart, liver and kidney
dysfunction. IV. Patients with previous history of uterine surgery.
Methods
Instruments and reagents
Rabbit Anti-Human SMARCE1 and CRISP3 monoclonal antibodies were purchased from Abcam Corporation, USA, and the immunohistochemistry kits were purchased from ZSGB-BIO Co. Ltd.,
Beijing, China.
Detection method
SP (streptavidin-perosidase) immunohistochemical method
was used to detect the immunoreactivity of SMARCE1 and CRISP3
proteins. Fix the cervical tissues sample with formalin solution
(10%), embed them with paraffin, and cut the samples into 5μm
thin slices after dehydrated. 3% hydrogen peroxide was used to
block endogenous peroxidase for 30 minutes after EDTA antigen
repair solution was used to repair under high pressure and Rabbit
Anti-Human primary antibody of 1:500 concentration was added
for overnight with 4
oC. After being taken out overnight, the room
temperature was restored and being washed with phosphate buffer solution (PBS) and the Rabbit Anti-Human second antibody
was added and incubated at 37
oC for 30 minutes, and the DAB
chromogenic reagent kit was developed and thehematoxylin was
stained and then sealed. The whole SP immunohistochemical process was strictly in accordance with the operating procedures of
the instructions. During the test, PBS was used as the negative
control instead of the primary antibody.
Result determination
Five visual fields were randomly selected from each section under high power microscope for observation, and the percentage
of positive cells and the staining intensity of cells were judged. The
positive signals of SMARCE1 and CRISP3 proteins were located in
the cytoplasm, and the positive cells were brown yellow or brown
granules. I. According to the staining intensity of positive cells, it
is judged that: colorless was 0 score, light yellow (weak positive)
was 1 score, brown yellow (medium intensity) was 2 score, brown
(strong positive) was 3 score. II. According to the percentage of
positive cells, positive cells accounted for 0% was 0 score, positive cells ≤ 10% was 1 score, positive cells accounted for 10%
~50%
was 2 score, positive cells accounted for 50%
~75% was 3 score,
positive cells accounted for>75% was 4 score. III. Staining index
=staining intensity+proportion of positive cells. negative expression was staining index was 0 score and positive expression was
staining index was ≥ 3 [10].
Detection of tumor markers
Took 5 ml of fasting peripheral venous blood from all subjects
in the morning, centrifuged for 10min at a rate of 3500 r/min,
and placed in a refrigerator at -80
oC temperature for testing. The
level of serum CEA, CA125 and CA153 were measured by electrochemiluminescence immunoassay. The detection instrument
was the automatic electrochemiluminescence immunoanalyzer
of Roche, Germany. The Kits were purchased from Beijing Lidman
Biochemical Co., Ltd., China and operated strictly according to the
instructions.
Statistical methods
The statistical analyses were performed using the Statistical
Package for the Social Sciences version 20.0 (SPSS Inc., Chicago, IL,
USA), and the counting data were chi-square test or rank sum test
for comparison. The measurement data were expressed by mean
± standard deviation ( ±s) with t-test for comparison. The area
under the Receiver Operating Characteristic (ROC) curve (AUC)
was used to analyze the diagnostic value of each parameter. The
difference was statistically significant. P ≤0.05 was considered statistically significant.
±s) with t-test for comparison. The area
under the Receiver Operating Characteristic (ROC) curve (AUC)
was used to analyze the diagnostic value of each parameter. The
difference was statistically significant. P ≤0.05 was considered statistically significant.
Table 1: Comparison of SMARCE1 and CRISP3 expression between
the two groups, cases (%).
| Group |
Case |
SMARCE1 |
CRISP3 |
| Positive |
Negative |
Positive |
Negative |
| Control group |
30 |
14 (46.67) |
16 (53.33) |
11 (36.67) |
19 (63.33) |
| Observation group |
50 |
38 (76.00) |
12 (24.00) |
31 (62.00) |
19 (38.00) |
| χ2 |
|
7.092 |
4.825 |
| P |
|
0.008 |
0.028 |
Table 2: The relationship of the expression of SMARCE1 and CRISP3 in cervical cancer tissues and the clinicopathological characteristics of patients (Cases).
| Group |
Case |
SMARCE1 |
CRISP3 |
| Positive |
χ2 |
P |
Positive |
χ2 |
P |
| Age |
|
|
0.005 |
0.943 |
|
0.198 |
0.656 |
| ≥45 |
35 |
27 |
|
|
21 |
|
|
| <45 |
15 |
11 |
|
|
10 |
|
|
| Lymph node metastasis |
|
|
1.576 |
0.209 |
|
2.972 |
0.085 |
| Yes |
18 |
16 |
|
|
14 |
|
|
| No |
32 |
22 |
|
|
17 |
|
|
| Degree of tumor differentiation |
|
|
6.255 |
0.0.012 |
|
4.089 |
0.043 |
| Highly differentiated |
20 |
11 |
|
|
9 |
|
|
| Medium and low differentiation |
30 |
27 |
|
|
22 |
|
|
| TNM staging |
|
|
1.882 |
0.170 |
|
2.266 |
0.132 |
| Stage I |
36 |
25 |
|
|
23 |
|
|
| Stage II and III |
14 |
13 |
|
|
8 |
|
|
Results
Comparison of SMARCE1 and CRISP3 expression
The positive expression rates of SMARCE1 and CRISP3 in the
observation group were significantly higher than the control
group (P<0.05). As shown in Table 1
The relationship of the expression of SMARCE1 and CRISP3 in
cervical cancer tissues and the clinicopathological characteristics
of patients
There was no significant difference in the positive expression
of SMARCE1 and CRISP3 among the age, lymph node metastasis
and TNM stage of cervical cancer patients (P>0.05). The positive
expression rates of SMARCE1 and CRISP3 were significantly different among different tumor differentiation degrees of cervical
cancer patients (P<0.05) and the lower the tumor differentiation
degree, the higher the positive expression rates of SMARCE1 and
CRISP3 proteins (P<0.05). As shown in Table 2.
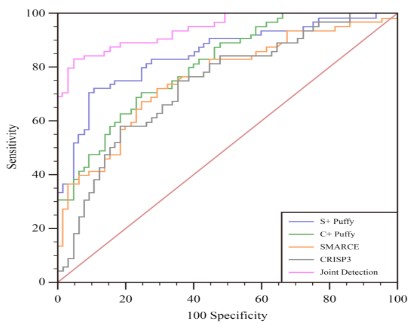
ROC curve of the clinical value of SMARCE1, CRISP3 combined
with tumor markers in the diagnosis of cervical cancer
The ROC curve results shown that the AUC of SMARCE1,
SMARCE1+tumor marker, CRISP3, CRISP3+tumor marker,
SMARCE1, CRISP3 combined with tumor marker for diagnosis of
cervical cancer were 0.760, 0.851, 0.739, 0.810 and 0.944 respectively. As shown in Table 4 and Figure 1.
Table 3: Comparison of serum tumor markers.
| Group |
Case |
CEA (ng/mL) |
CA153 (U/mL) |
CA125 (U/mL) |
| Control group |
30 |
6.24 ± 2.02 |
15.46 ± 3.46 |
66.28 ± 8.84 |
| Observation group |
50 |
2.85 ± 0.90 |
6.84 ± 2.12 |
41.34 ± 6.76 |
| t |
|
8.673 |
12.312 |
13.285 |
| P |
|
0.000 |
0.000 |
0.000 |
Table 4: ROC curve of clinical value of SMARCE1, CRISP3 combined
with tumor markers in diagnosis of cervical cancer.
| Screening method |
95%CI |
AUC |
Specificity (%) |
Sensitivity (%) |
| CRISP3 |
0.655-0.812 |
0.739 |
64.62 |
75.38 |
| SMARCE1 |
0.678-0.831 |
0.760 |
72.31 |
70.77 |
| CRISP3+ tumor markers |
0.732-0.873 |
0.810 |
75.38 |
70.77 |
| SMARCE1+ tumor markers |
0.778-0.908 |
0.851 |
89.23 |
72.31 |
| SMARCE1, CRISPS
Combined tumor markers |
0.889-0.977 |
0.944 |
95.38 |
83.08 |
Discussion
In recent years, with the change of people's life and eating
habits, the incidence of cervical cancer has been increasing year
by year [11]. The main clinical picture of patients with cervical
cancer are thin liquid discharge like water, earthy smell of leucorrhea, irregular vaginal bleeding, anemia, algopareunia and other
clinical symptoms which have a serious impact on the patient's
reproductive function and life safety and health] [12]. Cervical
cancer is also known as Invasive Carcinoma of Cervix. Cervical intraepithelial neoplasia is the early stage of cervical cancer, also
known as Precancerous Lesion Phase [13]. According to clinical
studies, patients with cervical cancer have a long Precancerous
Lesion State, and it takes about 5-10 years to develop from cervical intraepithelial neoplasia to cervical cancer [14]. Therefore,
early detection and diagnosis of cervical intraepithelial neoplasia
and cervical cancer, and active treatment of precancerous lesions
can effectively reduce the incidence and mortality of cervical cancer and improve the quality of life of patients with cervical lesions.
In clinical screening and diagnosis of cervical cancer with vinegar
white test combined with iodine test, colposcopy, HPV screening,
cervical smear cytology, cervical and cervical tube biopsy, cervical conization screening [15]. The emergence of various screening
technologies has improved the detection rate of clinical cervical
cancer, but the screening costs of various screening methods are
different, and the sensitivity and specificity are different. In recent
years, with the development of clinical testing technology, the detection and diagnosis of cancer patients using molecular marker
detection has gradually become a perspective study trend.
It was found that human SWI/SNF chromatin-remodeling
complex consists of 9~12 subunits, and SMARCE1 was one of the
subunits of human SWI/SNF chromatin remodeling complex [16].
The human SWI/SNF chromatin-remodeling complex contains
one of the ATPases of the SMARCA4 or SMARCA4 and three major core subunits and other complex specific variant subunits.
The subunits together played biological roles in regulating cell
cycle progress, differentiation, DNA repair, activation, genomic
instability, and programmed cell death [17]. Zhang Li, et al. [18]
found that SMARCEI was a specific and sensitive marker of clear cell meningioma, and SMARCEI mutation could lead to the occurrence of clear cell meningioma. SMARCEI mutation causes the
loss of SMARCEI function, leading to the loss of inhibition of SWI/
SNF complex on tumor and participating in the occurrence and
development of tumor [19]. The results of the study shown that
the positive expression rates of SMARCE1 and CRISP3 in the observation group were significantly higher than the control group.
It was indicated that SMARCEI was expressed in cervical cancer
patients and the abnormal expression of SMARCEI maid participate in the occurrence and development of cervical cancer. The
results of the study also found that the positive expression rate of
SMARCE1 was statistically significant in different tumor differentiation degrees of cervical cancer patients, and the lower the tumor
differentiation degree, the higher the positive expression rate of
SMARCE1 and CRISP3 proteins. It was indicated that the abnormal
expression of SMARCEI may have an impact on the pathological
changes of cervical cancer and may play a key role in promoting
the carcinogenesis and development of cervical cancer.
Human CRISP3 is located on human chromosome 6 and is the
third member of the cysteine rich secretory protein family and is
widely distributed in human tissues. It is detected in human body
fluid secretion including sweat, plasma, prostate, pancreas and
salivary glands [20]. The study found that CRISP3 is low expressed
in colon, thymus, ovary and epididymis tissues, but its specific
function has not been clearly studied [21]. CRISP3 is also low
expressed in various tumor tissues that Henriksen R, et al [22].
found that CRISP3 is low expressed in malignant ovarian epithelial
cells. Volpert M, et al. [23] found that CRISP3 can be used as a
prognostic marker of prostate cancer. The higher the expression
level of CRISP3 in prostate tissue, the higher the risk of recurrence
of prostate cancer patients. WANG Y, et al. [24] found that the
detection of CRISP3 level may be a new method to predict breast
cancer. The low expression of CRISP3 in breast cancer patients is
related to the overall survival rate and disease-free survival rate.
The results of the study shown that the positive expression rate of
CRISP3 in the observation group was significantly higher than the
control group. It is indicated that CRISP3 is expressed in patients
with cervical cancer and the abnormal expression of CRISP3 may
participate in the occurrence and development of cervical cancer.
The results of the study also shown that the positive expression
rate of CRISP3 was statistically significant in different tumor differentiation degrees of cervical cancer patients, and the lower the
tumor differentiation degree, the higher the positive expression
rate of SMARCE1 and CRISP3 proteins. The abnormal expression
of CRISP3 may have an impact on the pathological changes of cervical cancer, and may play a key role in promoting the carcinogenesis and development of cervical cancer.
Tumor markers refer to proteins, peptides or other biological
substances are produced by the body in the process of tumor occurrence, development, invasion and metastasis of tumor cells
which are synthesized, secreted or shed into body fluids or tissues
by the tumor cells or the body in response to tumor cells [25].
The content of tumor markers in normal healthy people is extremely low, but it is obviously expressed at a high level in tumor
tissues. Therefore, the determination of tumor markers presence
or content could be used to diagnose the generation of malignant
tumors, analyze the patient's condition, monitor metastasis, and
judge the prognosis of patients [26]. CEA is an acid glycoprotein
isolated from embryonic colon mucosa and colon adenocarcinoma which is expressed on the surface of tissue cell membrane
and is widely used in the differential diagnosis of malignant tumors [27]. CA125 is a mucin-like glycoprotein with high molecular weight which can promote cell metastasis and infiltration by
influencing mutual recognition and adhesion among cells [28].
CA153 is a polymorphic epithelial mucin secreted by glands and
exists in many kinds of adenocarcinoma. Studies have found that
the increase rate of CA153 can reach about 70% when tumor cells
metastasize so that it has good diagnostic value for the development and prognosis of the disease [29]. The results of the study
shown that the level of the serum CEA, CA125, CA153 in the observation group were significantly higher than the control group.
It is indicated that CEA, CA125 and CA153 are highly expressed in
cervical cancer patients, and the changes are related to the occurrence and development of cervical cancer.
In addition, the study results also found that the ROC curve analysis showed that the AUC values of SMARCE1, SMARCE1+tumor
markers, CRISP3, CRISP3+tumor markers, SMARCE1 and CRISP3
combined tumor markers in the diagnosis of cervical cancer were
0.760, 0.851, 0.739, 0.810, 0.944, respectively. It is indicated that
the combined detection of SMARCE1 and CRISP3 combined tumor markers has high clinical diagnostic value for cervical cancer.
The study has the following deficiencies including only a small
sample, single center study, and does not clarify how SMARCE1
and CRISP3 participate in the occurrence and development of cervical cancer. Large sample, multi-center studies are still needed
in the future, and more in-depth biological research is needed to
further clarify the relevant pathways.
Conclusion
To sum up, SMARCE1 and CRISP3 are expressed in cervical cancer patients, CEA, CA125 and CA153 are highly expressed in the
serum of cervical cancer patients, and the combined detection of
SMARCE1, CRISP3 and tumor markers has high clinical diagnostic
value for cervical cancer.
References
- Jing Z, Wenfan Z, Jinhao Y. Correlation and clinical analysis of serum tumor markers and endocrine hormones in patients with cervical cancer. Journal of Clinical Laboratory. 2021; 39: 679-682.
- Pingcan Y, Wei W. Study on HPV Genotyping and Serum tumor
marker Detection in the diagnosis of Cervical cancer. 2021; 8: 66-
69
- Ziyu M, Yifei Z, Zhenhua L. Expression of PD-L1 and tumor infiltrating lymphocyte markers in uterine cervical carcinoma. Chinese
Journal of Pathology 2022; 51: 602-607.
- Liu H, Zhao YR, Chen B, Ge Z, Huang JS, et al. High expression of
SMARCE1 predicts poor prognosis and promotes cell growth and
metastasis in gastric cancer. Cancer Manag Res 2019; 11: 3493-509.
- Wu HJ, Zhuo Y, Zhou YC, Wang XW, Wang XP, et al. miR-29a promotes hepatitis B virus replication and expression by targeting
SMARCE1 in hepatoma carcinoma. World J Gastroenterol. 2017;
23: 4569-4578.
- Wu H, Wei HY, Chen QQ. Long noncoding RNA HOTTIP promotes
the metastatic potential of ovarian cancer through the regulation
of the miR-615-3p/SMARCE1 pathway. Kaohsiung J Med Sci. 2020; 36: 973-982.
- Noh BJ, Sung JY, Kim YW, Chang SG, Park YK, et al. Prognostic value
of ERG, PTEN, CRISP3 and SPINK1 in predicting biochemical recurrence in prostate cancer. Oncol Lett. 2016; 11: 3621-3630.
- Chen L, Zhang E, Guan J, Chen Z, Ye J, et al. A Combined CRISP3 and
SPINK1 Prognostic Grade in EPS-Urine and Establishment of Models to Predict Prognosis of Patients With Prostate Cancer. Front
Med (Lausanne) 2022; 9: 832415.
- Qi Z, Xiaohua W, Jihong L. Guidelines to the diagnosis and treatment of cervical cancer (4th edition). Chinese Journal of Practical
Gynecology and Obstetrics. 2018; 34: 613-622.
- Hatimihan Maimaiti, Gulikezi MAIMAITIREXIAT, Yuting L. Expression of CBX7 protein in cervical cancer and its clinical significance.
Basic and Clinical Medicine 2022; 42: 721-725.
- Yuqiang Z, Jie L, Qing G. Expression and clinical significance of
CDK9 and iASPP in cervical carcinoma. Journal of Guangdong Pharmaceutical University. 2022; 38: 89-94.
- Guo M, Liang L, Wu L, Xie D, Li J, et al. Application Value of RealTime Ultrasonic Elastograph with Serum Human Epididymis Protein 4, Interleukin-33, and Carbohydrate Antigen 153 in Diagnosis
of Early Cervical Cancer. J Healthc Eng. 2022; 2022: 4880874.
- Gao L, Lv J, Hou L, Yuan Y, Wan Q, et al. Clinical Effects of Chinese
Herbal Decoction Combined with Basic Chemoradiotherapy and
Nursing Intervention in the Treatment of Cervical Cancer and the
Effect on Serum CEA, CA125, and TNF-alpha Levels. Evid Based
Complement Alternat Med. 2021; 2021: 1446864.
- Zhengtong H. Analysis of the Relationship Between the Expression
of Notch Pathway Protein and the Effectiveness of Cisplatin Based
Uterine Artery Embolization in Locally Advanced Cervical Cancer.
The Practical Journal of Cancer. 2022; 37: 1100-1104.
- Yawen. L, Lingling. Z, Benhui. H. Expression of TRIM37 in Cervical
Cancer and Clinical Significance. The Practical Journal of Cancer.
2022; 37: 1092-1095.
- St Pierre R, Collings CK, Same Guerra DD. SMARCE1 deficiency generates a targetable mSWI/SNF dependency in clear cell meningioma. Nat Genet. 2022; 54: 861-873.
- Tauziede-Espariat A, Parfait B, Besnard A, Lacombe J, Pallud J, et al.
Loss of SMARCE1 expression is a specific diagnostic marker of clear
cell meningioma: a comprehensive immunophenotypical and molecular analysis. Brain Pathol. 2018; 28: 466-474.
- Zhang L, Yao ZG, Lian F, Wang DZ, Chen YP, et al. The role of
SMARCE1 in the diagnosis of clear cell meningioma. Zhonghua
Bing Li Xue Za Zhi. 2020; 49: 234-238.
- Sethuraman A, Brown M, Seagroves TN, Wu ZH, Pfeffer LM, et
al. SMARCE1 regulates metastatic potential of breast cancer cells
through the HIF1A/PTK2 pathway. Breast Cancer Res. 2016; 18: 81.
- Leng D, Miao R, Huang X, Wang Y. In silico analysis identifies CRISP3
as a potential peripheral blood biomarker for multiple myeloma:
From data modeling to validation with RT-PCR. Oncol Lett. 2018;
15: 5167-5174.
- Pathak BR, Breed AA, Deshmukh P, Mahale SD. Androgen receptor mediated epigenetic regulation of CRISP3 promoter in prostate
cancer cells. J Steroid Biochem Mol Biol. 2018; 181: 20-27.
- Henriksen R, Lundwall A, Udby L, Fernlund P. The expression of
beta-microseminoprotein but not CRISP3 is reduced in ovarian cancer and correlates to survival. Anticancer Res. 2012; 32: 3993-
3999.
- Volpert M, Furic L, Hu J, O’Connor AE, Rebello RJ, et al. CRISP3 expression drives prostate cancer invasion and progression. Endocr
Relat Cancer. 2020; 27: 415-430.
- Wang Y, Sheng N, Xie Y, Chen S, Lu J, et al. Low expression of CRISP3
predicts a favorable prognosis in patients with mammary carcinoma. J Cell Physiol. 2019; 234: 13629-1338.
- Ying L. Clinical significance of tumor markers combined with TCT
and HPV DNA detection in cervical cancer and precancerous lesions. Modern Joumal of Integrated Traditional Chinese and westem Medicine. 2016; 25: 92-3,108.
- lei Z, fanqing Z. Study on Combined Detection of SCC-Ag,CEA and
CA125 in the Diagnosis of Cervical Cancer. Chin J Lab Diagn. 2017;
21: 1708-1710.
- Meng H, Zhang Y, Chen Y. Diagnosis Value of Colposcope Combined with Serum Squamous Cell Carcinoma Antigen, Carbohydrate
Antigen 125, and Carcinoembryonic Antigen for Moderate to Advanced Cervical Cancer Patients Treated with Modified Fuzheng
Peiyuan Decoction. Evid Based Complement Alternat Med. 2021;
2021: 4355805.
- Xiaofeng W, Xianfeng W, Chunyan T. Clinical research on the detection of serum tumor markers in patients with cervix cancer.
Chinese Journal of Health Laboratory Technology. 2017; 27: 63-65.
- Lin. Q, Liji L, Meige. Application of tumor markers in the diagnosis
of cervical cancer. Chongqing Medicine 2017; 46: 4425-4427.