Introduction
Metastasis and recurrence are two inevitable and thorny problems in cancer treatment. Photodynamic therapy (PDT) is considered the first-line therapy for the treatment of oligometastatic
recurrent endotracheal malignancies smaller than 10 mm without
extrachondral spread [1], and immunotherapy is also playing an
increasingly important role, but both have their own limitations.
Recent studies have shown that PDT combined with programmed death receptor 1 (PD-1) / programmed cell death-Ligand 1
(PD-L1) inhibitors performs better than single treatment modalities [2-6]. These studies are still in the laboratory stage, clinical data is still limited. Here, we report a case of a patient with
advanced bronchial tumor who achieved complete remission
(CR) after PDT combined with a PD-1 inhibitor and was free of
recurrence and metastasis during a 14-month follow-up period.
Case report
On February 23, 2021, a 69-year-old male patient presented
to our hospital with a history of cough and sputum production
for more than 5 months. He had experienced hypertension for
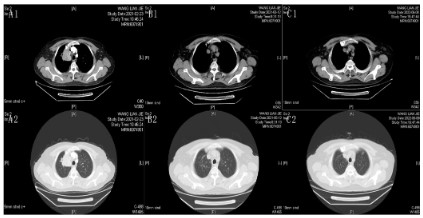
10 years and had a 40-year history of smoking. Contrast Computed Tomography (CT) revealed a paramediastinal occupancy in
the upper lobe of the right lung (Figure 1A1, A2). Considering the
possibility of lung cancer and mediastinal lymph node metastasis,
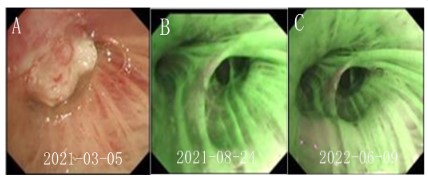
bronchoscopy revealed a neoplastic opening in the upper lobe of
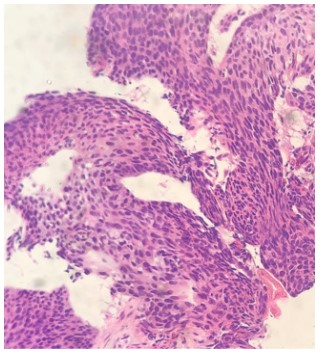
the right lung (Figure 2A), and pathology of biopsy samples confirmed squamous cell carcinoma (Figure 3). Immunohistochemistry
findings were as follows: CK5/6 (+), p40 (+), ALK-D5F3 (-), ALK-D5F3-N (-), PD-L1-22C3 (TPS: -). Genetic testing was performed
using amplification refractory mutation system (ARMS)-PCR, revealing no mutations in EGFR, ALK, ROS1, KRAS, or HER2. Ultrasound examination, bone imaging, or cranial magnetic resonance
imaging revealed no metastasis. The patient was diagnosed with
squamous cell carcinoma of the right lung (cT4N2M0, stage IIIB).
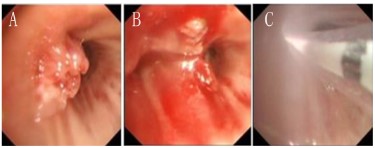
Bronchoscopic resection of the lesion was performed on March
15, 2021 (Figure 4 A,B), and the first cycle of chemotherapy was
administered on March 17, 2021. However, the second cycle of
chemotherapy was discontinued owing to elevated transaminase
levels. After obtaining patient consent and excluding contraindications, the first PDT was performed on April 14, 2021 (Figure
4C). Forty-eight hours before laser illumination, hematoporphyrin
(ChongQing Milelonge Biopharmaceutical Co. Ltd.) was administered intravenously at 2 mg/kg. A 3 cm diffusion segment length
columnar fiber (OPTIGUIDE; Pinnacle Biologics; wavelength=630
nm) was introduced through the bronchoscope to irradiate the
mucosal infiltrate of the anterior segment of the right upper lobe
opening using an energy density of 150 J/cm2 (250 mW×1200 s);
the same was performed the following day. Light avoidance was
strictly enforced four weeks post-surgery, and no complications
occurred. Adjuvant chemotherapy was administered one-month
post-PDT. Chest CT examination 2 months after PDT suggested
CR (Figure 1B1,B2). However, the patient had recurrent myelo-suppression after receiving the second and third cycles of post-operative chemotherapy. After comprehensive consideration and
with the patient’s consent, we administered maintenance therapy
with sindilizumab for the patient. During the 14-month follow-up
period, no tumor recurrence or metastasis was detected (Figure
1C1,C2, and 2C). The patient is currently on maintenance treatment with sindilizumab.
Discussion
PDT has become an important alternative treatment modality for inoperable bronchial tumors, and a large body of data
demonstrates the superiority of PDT in local treatment of bronchial tumors compared to other alternative treatment modalities
in terms of efficacy and side effects [7-10]. On the other hand,
immunotherapy also has important clinical applications in cancer
treatment. However, both therapies have their own limitations:
The cytotoxic effect of PDT is limited by the spatial limitation of
the photoactivation process, which limits the direct effect of PDT
on the therapeutic field, while the effect of immunotherapy often fails due to the lack of immunogenicity of tumor tissue. Thus
monotherapies cannot meet the current needs of cancer treatment. PDT has been shown to modulate the antitumor immune
response, activate antigen-presenting cells, and reprogram the
tumor microenvironment to more easily target immune check-points. Therefore, Immune Checkpoint Inhibitors (ICI) combined
with PDT has been developed as a new therapeutic option to help
enhance the antitumor immune response and improve the efficacy of preventing tumor metastasis and tumor recurrence.
PD-1/ PD-L1, as an important immune checkpoint, has been
shown to be upregulated during PDT [11,12]. However, the potential mechanism remains unknown. Adjuvant IL6 produced by PDT-induced tissue damage can augment PD-1 expression through
STAT3 [13] and PD-L1 expression and stability through JAK1 [14].
PDT also induces PD-L1 production by increasing T-cell infiltration
and interferon (IFN)-γ expression [11,15]. In addition, PDT may
cause transient hypoxia in the tumor microenvironment, which
in turn potentially alters tumor and immune cell phenotypes
through upregulation of PD-L1 that is dependent on HIF-1α signaling [16-18,12].
Recently, several in vivo experimental studies have shown that
PDT combined with PD-1/PD-L1 checkpoint blockade is more effective than monotherapy against tumors. Bao et al. observed in
an in vivo experiment that PDT targeting tumor vasculature suppressed the growth of subcutaneous 4T1 tumor and inhibited its
lung metastasis, tumor PD-L1 levels were also markedly increased
after PDT. In combination with an anti-PD-1/PD-L1 blockade further enhances the tumor suppression effect [11]. The enhanced
permeability and retention (EPR) effect of nanoparticles allows
for more selective accumulation of photosensitizers in tumor
tissue, and third-generation PDT photosensitizers using nanoparticle platforms have also been studied in combined with immune
checkpoint blockade. Duan et al. combined the photosensitizer
with zinc pyrophosphate (ZnP@pyro) nanoparticles, which significantly inhibited in situ 4T1 tumor growth after 670 nm light activation. When combined with a PD-L1 blocker, ZnP@pyrolipPDT
also prevented spontaneous lung metastasis [19]. In contrast to
the conventional treatment strategy achieved by intravenous injection of anti-PD-1/PD-L1 antibodies in combination with PDT, Liu
et al. assembled anti-PD-L1 antibodies and photosensitizer into
nanoparticles (BDP-I-N-anti-PD-L1). Active targeting by immune
checkpoint antibodies and EPR effect of nanoparticles further
achieved effective accumulation of tumor tissues and eliminated
MC38 mouse colon tumors by synergistic effect of PDT and ICB,
generating immune memory to prevent tumor recurrence with
good biosafety [20]. RGDyK as a novel PD-L1 inhibitor, Zhou et
al. prepared RGDyK-modified nanoparticles (ZnPP@MSN-RGDyK).
ZnPP@MSN-RGDyK nanoparticles precisely targeted β3-int to inhibit PD-L1 and showed high photodynamic treatment efficiency
and excellent immunotherapeutic effects in a mouse model of NSCLC-spinal metastasis [21]. Chen et al. synthesized a nanoparticle
loaded with PDT porphyrin and interfering RNA (siRNA), which
combined photodynamic therapeutic ability and mediated PD-L1
gene silencing to achieve superior anti-tumor effects [22]. Wang
et al. rationally designed a multifunctional micelle by integrating
acid-activated cationic micelles, photosensitizers, and small siRNA. PDT or siRNA-mediated PD-L1 gene silencing with a 671-nm
laser alone significantly inhibited the growth of more than 60% of
B16-F10 melanomas, but the combination of the two completely
inhibited tumor growth [23].
Clinical data for PDT combined with PD-1/PD-L1 checkpoint
blockade therapy is scarce [6]. Wang et al. reported a patient
with advanced esophageal cancer, who had been treated with
two cycles of chemotherapy at the local hospital but failed. In this
case, after metal stent implantation, the patient underwent a remarkable and successful treatment of PDT combined with sintilimab, a PD-1 inhibitor. An additional immune checkpoint inhibitor
and chemotherapy offer the opportunity to eliminate residual and invisible tumors. The patient had an excellent prognosis that
not only the primary lesion was cured, but also the metastatic
lymph nodes were significantly reduced, with no tumor recurrence in the last endoscopic review [24]. Maller et al. reported
a patient with squamous lung cancer (T2aN0M0) exhibiting recurrence after right upper lung lobectomy. After multiple PDT
treatments, the authors found that lesions were still detectable
in the tracheal ridge and proximal left main stem bronchus, and
positron emission tomography (PET)-CT revealed bone metastases. Furthermore, immunohistochemical analysis of the biopsy
specimens indicated that PD-1 was highly expressed. Accordingly,
the authors treated the patient with intravenous pembrolizumab
(200 mg every three weeks). Three months later, tracheoscopy
showed CR, PET-CT revealed CR of all previously hypermetabolic
areas, and no recurrence was detected during the 1-year follow-up period. This previous case report and our patient suggest that
post-PDT combined with a PD-1 inhibitor could be a potential
treatment strategy to increase survival benefits in patients with
advanced lung cancer [25].
In April 2021, Roswell Park Cancer Institute initiated a Phase I
clinical trial to evaluate the ability of PDT to amplify immunotherapy responses in patients with non-small cell lung cancer with
pleural disease (NCT number: NCT04836429). Sixteen patients
are expected to be enrolled. This trial evaluates the side effects
of intraoperative photodynamic therapy to enhance ICI drug response. The incidence of serious adverse events (SAEs) was determined by recording the occurrence of SAEs during the first 28
days of poststudy-related immunotherapy. In addition, patients
are followed over a two-year time frame for progression-free survival (PFS), overall survival (OS), immunophenotypic changes in
peripheral blood CD8+ T cells, and changes in platelet-lymphocyte
ratios. The study is expected to be completed in December 2023
[6].
Although many preclinical studies have shown that nanotechnology-based Photodynamic Immunotherapy (PDIT) can enhance
immunity against both primary and metastatic tumors. However,
the effectiveness of such nanotechnology-based PDIT has not yet
been clinically validated. The safety of nanomaterials is a major
challenge for the future of nanophotodynamic immunotherapy.
However, cellular and animal experiments have been mainly
conducted, and human experimental data are scarce. A large
number of human toxicity experiments must also be performed
before clinical application.
Conclusion
In conclusion, PDT combined with PD-1/PD-L1 checkpoint
blockade can not only eradicate primary tumors, but also control
metastatic tumors. However, there is a lack of sufficient clinical
data and large-cohort studies are still needed. PDIT has the potential to move forward as a next-generation cancer treatment
technology. However, human toxicity trials are needed to validate
efficacy and safety before clinical application.
References
- Singh H, Benn BS, Jani C, Abdalla M, Kurman JK. Photodynamic
therapy for treatment of recurrent adenocarcinoma of the lung
with tracheal oligometastasis. Respir Med Case Rep. 2022; 37:
101620.
- Cramer GM, Moon EK, Cengel KA, Busch TM. Photodynamic Therapy and Immune Checkpoint Blockade(†). Photochem Photobiol.
2020; 96: 954-961.
- Hua JP, Wu L, Gan Z, Zhang J, He, et al. Current Strategies for Tumor
Photodynamic Therapy Combined With Immunotherapy. Front
Oncol. 2021; 11: 738323.
- Kleinovink JW, Ossendorp F. Combination of Photodynamic Therapy and Immune Checkpoint Blockade. Methods Mol Biol 2022;
2451: 589-596.
- Zhang Q, Li L. Photodynamic combinational therapy in cancer
treatment. J buon. 2018; 23: 561-567.
- Zhao Y, Liu X, Liu X, Yu J, Bai X, et al. Combination of phototherapy
with immune checkpoint blockade: Theory and practice in cancer.
Front Immunol. 2022; 13: 955920.
- Chhatre S, Murgu S, Vachani A, Jayadevappa R. Photodynamic therapy for stage I and II non-small cell lung cancer: A SEER-Medicare
analysis 2000-2016. Medicine (Baltimore). 2022; 101.
- Chhatre S, Vachani A, Allison RR, Jayadevappa R. Survival Outcomes with Photodynamic Therapy, Chemotherapy and Radiation
in Patients with Stage III or Stage IV Non-Small Cell Lung Cancer.
Cancers (Basel). 2021; 13.
- Yi E, Chang JE, Leem C, Kim S, Jheon S. Clinical results of photodynamic therapy in tracheobronchial malignancy. J Photochem Photobiol B. 2016; 156: 56-60.
- Zhang Q, Zheng K, Gu X, Gao Y, Zhao S, et al. Photodynamic therapy for primary tracheobronchial malignancy in Northwestern
China. Photodiagnosis Photodyn Ther. 2022; 37: 102701
- Bao R, Wang Y, Lai J, Zhu H, Zhao Y, et al. Enhancing Anti-PD-1/PD-L1 Immune Checkpoint Inhibitory Cancer Therapy by CD276-Targeted Photodynamic Ablation of Tumor Cells and Tumor Vasculature.
Mol Pharm. 2019; 16: 339-348.
- Yuan Z, Fan G,Wu H, Liu C, Zhan Y, et al. Photodynamic therapy
synergizes with PD-L1 checkpoint blockade for immunotherapy of
CRC by multifunctional nanoparticles. Mol Ther. 2021; 29: 2931-
2948.
- Austin JW, Lu P, Majumder P, Ahmed R, Boss JM. STAT3, STAT4,
NFATc1, and CTCF regulate PD-1 through multiple novel regulatory
regions in murine T cells. J Immunol. 2014; 192: 4876-4886.
- Chan LC, Li, CW, Xia W, Hsu JM, Lee HH, et al. IL-6/JAK1 pathway
drives PD-L1 Y112 phosphorylation to promote cancer immune
evasion. J Clin Invest. 2019; 129: 3324-3338.
- Xiong W, Qi L, Jiang N, Zhao Q, Chen L, et al. Metformin Liposome-Mediated PD-L1 Downregulation for Amplifying the Photodynamic Immunotherapy Efficacy. ACS Appl Mater Interfaces. 2021; 13:
8026-8041.
- Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014; 74: 665-674.
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor
cells in the tumor microenvironment. J Exp Med. 2010; 207: 2439-
2453.
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, et al. PD-L1
is a novel direct target of HIF-1α, and its blockade under hypoxia
enhanced MDSC-mediated T cell activation. J Exp Med. 2014; 211:
781-790.
- Duan X, Chan C, Guo N, Han W, Weichselbaum RR, et al. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles
Synergizes with Immune Checkpoint Blockade To Elicit Antitumor
Immunity and Antimetastatic Effect on Breast Cancer. J Am Chem
Soc. 2016; 138: 16686-16695.
- Liu Q, Tian J, Tian Y, Sun Q, Sun D, et al. Near-Infrared-II Nano-particles for Cancer Imaging of Immune Checkpoint Programmed
Death-Ligand 1 and Photodynamic/Immune Therapy. ACS Nano.
2021; 15: 515-525.
- Zhou L, Liang H, Ge Y, Ding W, Chen Q, et al. Precisely Targeted
Nano-Controller of PD-L1 Level for Non-Small Cell Lung Cancer
Spinal Metastasis Immunotherapy. Adv Healthc Mater. 2022; 11:
e2200938.
- Hao K, Lin L, Sun P, Hu L, Atsushi M, et al. Cationic Flexible Organic
Framework for Combination of Photodynamic Therapy and Genetic Immunotherapy Against Tumors. Small. 2021; 17: e2008125.
- Wang D, Wang T, Liu J Yu H, Jiao S, et al. Acid-Activatable Versatile
Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic
Immunotherapy. Nano Lett. 2016; 16: 5503-5513.
- Wang XY, Maswikiti EP, Zhu JY, Ma YL, Zheng P, et al. Photodynamic
therapy combined with immunotherapy for an advanced esophageal cancer with an obstruction post metal stent implantation: A
case report and literature review. Photodiagnosis Photodyn Ther.
2022; 37: 102671.
- Maller B, Kaszuba F, Tanvetyanon T. Complete Tumor Response of
Tracheal Squamous Cell Carcinoma After Treatment With Pembrolizumab. Ann Thorac Surg. 2019; 107: e273-e274.