Introduction
Hepatocellular Carcinoma (HCC) is a highly malignant tumor
of the digestive tract with a high mortality rate [1]. According to
recent surveys, the incidence of HCC has increased rapidly among
all cancers and the relative survival rate of HCC is much lower
than that of other cancers, except pancreatic cancer [2]. The high
recurrence and metastasis rate are important reasons for hardly
maintaining a long-term prognosis in patients with HCC [3]. Although HCC treatment strategies have improved dramatically
with the rapid development of medical technology, current research on the treatment of HCC remains a hot spot for all humans
[4]. In particular, studies have shown that various mechanisms in
the microenvironment of HCC can improve the ability to escape
and tolerate immune responses and decrease effector function
of immune cells [5,6]. Therefore, it is imperative to clarify the microenvironment of HCC.
HCC is characterized by uncomplicated distant metastases and
rapid progression, mainly considering the blood supply of HCC tissues [7]. Angiogenesis is an early characteristic of solid tumors,
which refers to how pre-existing endothelial cells form new blood
vessels under suitable conditions [8]. Furthermore, angiogenesis
plays a facilitating factor for tumor growth, progression, invasion,
and metastasis. Tumor and neovascular ECs may form a highly integrated system that can promote each other’s development [9].
Tumor cells stimulate the proliferation of vascular ECs by secreting specific cytokines, and ECs can also provide rich oxygen and
nutrients to promote tumor cells growth [10]. Vascular ECs are reported to be found in glioblastoma derived from tumor cells [11].
Furthermore, some ECs were found to have typical human chromosomes instead of murine chromosomes in mouse transplanted
tumors of human glioblastoma [12], suggesting that stem cell-like
tumor cells have tumor angiogenesis during tumor formation.
Thus, we predicted that angiogenesis inhibition is a potentially
effective strategy for HCC therapy.
ENG is mainly enriched on the surface of adult vascular ECs,
and its primary function is to promote tumor tissue angiogenesis
[13]. ECs cultured in vitro can detect a higher level of ENG expression during the proliferation process [14]. In tumor tissues, ENG
is highly expressed in the process of angiogenesis and vascular
remodeling [15-17], and other studies have confirmed that ENG is
a specific marker of angiogenesis [18]. Studies showed that ENG
was only expressed in the neovascular ECs of HCC tissues but not
in normal tissues [19]. Therefore, ENG can be used as a specific
target for identifying the neovascularization of HCC. Sorafenib, as
an anti-angiogenesis targeted drug, is one of the drugs currently
used in the treatment of advanced liver cancer. It acts mainly by
indirectly inhibiting the expression of VEGFR, PDGFR, and C-kit
expression, and then prevents the formation of tumor neovascularization [20-22]. However, the role of ENG in sorafenib inhibited
tumor progression is poorly understood.
In this article, we demonstrate that: (i) eight subpopulations
of tumor cells were found in HCC by single-cell RNA sequencing
assay, and tumor cells were retrodifferentiated to vascular endothelial-like cells; (ii) ENG, overexpressed in the progression of
tumor infiltration, predicted aggressive clinicopathological characteristics and poor prognosis; (iii) ENG expression could upregulate COL1A1 expression to promote HCC angiogenesis; (iv) ENG expression promoted the immune response and immune cell infiltration; (v) ENG expression promoted antitumor therapy of sorafenib in HCC patients. These data suggested that ENG expressed
in neovascular derived from tumor cells promoted antitumor therapy of sorafenib through activation of the immune response and
immune cell infiltration in HCC patients, which highlighted a new
scientific strategy for antitumor therapy of HCC.
Materials and Methods
Cell culture
HCC cells (Huh7 and HepG2) and HUVECs were obtained and
authorized from the China Center for Type Culture Collection and
were cultured in Dulbecco’s modified eagle medium DMED added
with 10% fetal bovine serum (Solarbio, Beijing, China), 100X Penicillin-Streptomycin (Solarbio). Huh7 cells were infected with
EGFP-Lv for stably exogenous expression of EGFP.
Patients and animals
Seventy-six paired tumor tissues, and adjacent HCC tissues
were collected from the Affiliated Hospital of Qingdao University from January 2020 to September 2021 (Table S1). Eighteen
HCC patients with sorafenib treatment, were collected for ENG
stain and CT films (Table S2). All of these studies were conducted under the supervision of the Research Ethics Committee
of the Affiliated Hospital of Qingdao University (Approval NO:
QYFYWZLL26589) and obtained informed consent forms from
patients or family members. Male Balb/c nude mice, aged five
weeks, were purchased from SPF (Beijing) Biotechnology Co., Ltd
and raised in an IVC system. EGFP-Lv infected Huh7 cells, 5x106,
were subcutaneously injected into mice for two weeks. All animal
experiments were approved and conducted under the supervision of the Affiliated Hospital of Qingdao University (Approval No:
QYFYWZLL26589).
Small animal ultrasound imaging system
The neovascularization density of tumor tissues was recorded
using the Small Animal Ultrasound Imaging System. Briefly, mice
anesthetized with pentobarbital sodium were fixed on a small animal ultrasound imaging system, and then a 40 MHz probe was
installed subsequently. After opening the software, the exposed
tumor site was wrapped with conductive glue. The image of neovascularization density was recorded by adjusting the probe to a
proper position.
Small animal in vivo laser confocal microscope
Shape and diameter of neovascularization were recorded on
Small Animal in vivo Laser Confocal Microscope. Briefly, pento-barbital sodium anesthetized mice were injected with 1% Evans
blue through the tail vein. A Probe-Based Confocal Laser was directly injected into tumor tissues for the neovascularization observation in vivo. Finally, an appropriate image was recorded.
Western blot and quantitative real-time polymerase chain
reaction (qRT-PCR)
Total protein was extracted with RIPA (Solarbio) from cells and
tissues. The extracted protein was separated with 8% SDS-PAGE
gels and transferred to the PVDF membrane immediately. After
blocking with 5% skim milk, PVDF membranes were blotted with anti-ENG (1:2000; Abcam), anti-COL1A1 (1:1000; Proteintech),
anti-CD31(1:1000; Sigma-Aldrich), anti-CD34(1:1000; Sigma-Aldrich), and anti-GAPDH (1:2000; Proteintech) antibodies and
incubated with HRP-linked secondary antibodies (1:5000; Proteintech), subsequently. After incubation with ECL solution, the
protein band was exposed and imaged by the Tanon-5200 chemiluminescence imaging system. Total RNA from 76 paired tumors
and adjacent tissues of HCC was isolated using Trizol reagent (In-vitrogen). qRT-PCR was performed using the SYBR Green PCR kit
(ABclonal) on an ABI Prism 7500 Sequence Detection system (Applied Biosystems). The primers used for the qRT-PCR analysis are
listed in Table S3.
Immunofluorescence assay
The frozen slices were fixed with 75% alcohol and penetrated
with 0.1% Triton X-100. After blocking with 1% goat serum, slices
were blotted with anti-EGFP (1:100; Abcam), anti-CD31(1:50;
Sigma-Aldrich), and anti-AFP (1:100; Sigma-Aldrich) antibodies.
The residual primary antibody was washed 1xPBS and the slices
were incubated with FITC or TRITC-linked secondary antibody at
1:200 dilution. Subsequently, 5 μl of DAPI solution was used for
DNA detection. After the 10 minutes of staining, the double immunofluorescence staining was photographed under laser scan
ning confocal microscopy.
Immunohistochemistry (IHC)
The immunohistochemistry assay was performed following the
manufacturer’s protocol (ZSGB, Beijing, China). First, tissue slices
were placed in a 56oC constant incubator for 30 min to dehydrate,
and then put them for hydration. After blocking with serum, 100
μL of primary antibody working liquor was supplemented and
cultivated at 37oC for 60 min. The second antibody was labeled
with goat anti-rabbit/anti-mouse IgG labeled with biotin. Subsequently, the horseradish enzyme-labeled streptavidin working liquor was supplemented and cultivated. An appropriate amount
of freshly prepared DAB solution was added to the tissue section
for 5 min. The slides were photographed and recorded under the
microscope.
Trans well cell migration experiment
HUVECs (Human Umbilical Vein Endothelial Cells) at logarithmic growth stage were used to suspend the cells in conditioned
medium and counted. Then, 200 ul of cell suspension was added
to the Trans well chamber, and 200 ul of medium containing 10%
FBS was added to the culture plate at the bottom of the chamber.
Posterior to the 12 or 48 h cultivation, cells on the chamber film
were subjected to fixation, dyeing, imaging, and the counting was
finished in 6 stochastic fields for each group via a microscopic device. Those assays were completed for three times.
HUVECs tubule formation experiment
Corning Matrigel was laid on the 96-well plate, and added 60
ul matrigel per hole. Then the 96-well plate was placed in the incubator for 30 min, and the matrigel was fully solidified. HUVECs
were suspended in conditioned medium, and then 1 × 104 HUVECs were inoculated in each well with 3 multiple wells in each
group. After incubating for 6 hours in the incubator, microscope
observation, photographing and counting were performed.
Data collection and analysis
Single cell RNA-seq data set (GSE149614) and nine HCC RNA-seq data sets were downloaded directly from GEO. Thirty-three
types of tumor RNA-seq data sets and clinicopathological characteristics were collected from the TCGA. The seurat R package was
used for the single-cell RNA-seq assay. Monocle2 R package was
performed for the analysis of pseudo-time trajectories to understand the fate of tumor cells. |logFoldChange| > 1 and P.Val < 0.05
were considered for the identification of differentially expressed
genes (DEGs) from ENG-highly and weakly expressed LIHC RNA-Seq from TCGA. Subsequently, Gene Set Enrichment Analysis
(GSEA) was performed using the MSigDB molecular signatures database and DEGs were identified. Estimate R package was used for
the estimation of the tumor microenvironment. CIBERSORT was
performed for immune infiltration in LIHC. Two-tailed Student’s t-tests were performed for differences among variables. A log-rank
test was performed for survival analysis. Spearman’s rank correlation was used to analyze the correlations of NEG with StromalS-core, ImmuneScore, ESTIMATScore, TumorPurity, immune infiltration, targets of sorafenib, and altered tumor diameter. Overall and
disease-free survival was analyzed with Log-rank tests. Data were
shown as the average ± SD. Statistically significant was shown as
P value <0.05.
Results
Retro differentiation of tumor cells to vascular endothelial-like cells in the process of infiltrating para-carcinoma
We all know that the type of HCC cell directly determines the
cancer progress [23]. However, the tumor cell progression in
HCC has not yet been elucidated. Therefore, we downloaded the
single-cell data set GSE149614 from GEO.
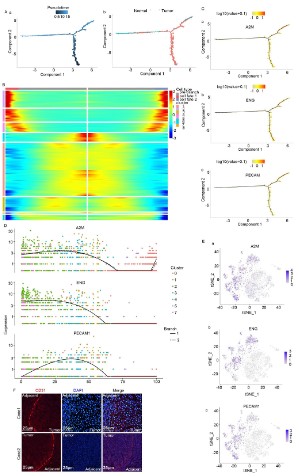
Subsequently, the cluster analysis indicated that there are
eight different subtypes of tumor cell (Figure S1A and B) distributed in HCC tumors and adjacent tissues, and each subtype of a
tumor cell has specific markers (Figure S1C and D). To understand
the evolution of tumor cells, pseudo-time analysis was performed. Interestingly, in the process of infiltration from the tumor to
adjacent tissue, tumor cells were retro differentiated with three
main distinct branch points (Figure 1A), and specific markers of
the subpopulation were reprogramed in this retro differentiation
process (Figure 1B). Interestingly, markers of vascular endothelial
cells, such as ENG, A2M, and PECAM1, were increased in the retro
differentiation process (Figure 1C-E). To further confirm the reliability, we performed immunofluorescence staining on samples
from HCC patients, and the result suggested that the vascular ECs
marker, CD31, highly expressed tumor cells at the tumor junction
(Figure 1F). All in all, these data showed that tumor cells were retro differentiated to vascular endothelial-like cells in the process
of invading peripheral normal tissues from the center of the HCC
tissues.
Transformation of HCC cells into neovascular endothelial cells
in a nude mouse model
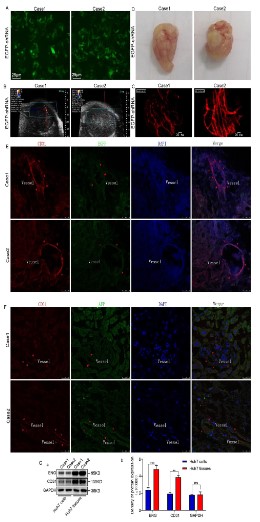
As shown above, tumor cells can transform into ECs in the process of outward invasion. To confirm the above results, we stably
introduced the exogenous EGFP gene into Huh7 cells by infecting
the EGFP-shRNA lentivirus (Figure 2A). Nude mice were subcutaneously injected with 5 × 106 Huh7 cells and then fed in the IVC system for two weeks. Subsequently, anesthetized nude mice
were performed under the S-Sharp Prospect small animal ultrasound imaging system for neovascular density, and a large amount
of microvascular was found in the subcutaneous tumor (Figure
2B). Consistently, neovascular enriched tumor tissues, pre-injected with 1% Evans blue, were further observed under in vivo laser confocal microscope (Figure 2C). The successfully constructed
subcutaneous tumor-bearing animal model is presented in Figure
2D. To further confirm the origin of neovascularity in tumor-bearing tissues, double immunofluorescence staining was performed
for exogenous EGFP and CD31, a marker of neovascularity, respectively. Approximately, tumor cells were highly expressed EGFP and
AFP, and endothelial cells were simultaneously expressed EGFP,
AFP, and CD31 (Figure 2E and F). Additionally, compared with tumor-bearing tissues, Huh7 cells expressed lower levels of ECs biomarker (Figure 2G). In summary, endothelial cells in neovascular
tumor tissues may be derived from the transformation of tumor
cells.
Vascular ECs biomarkers highly expressed in adjacent tumor
tissues
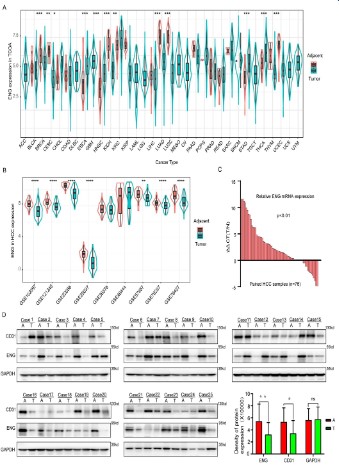
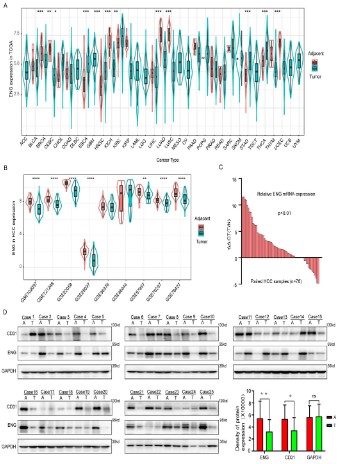
The above result suggested that microvascular cells were derived from tumor cells in HCC. Therefore, to clarify the expression
of the vascular ECs biomarker in HCC progression, transcriptome
data was collected, including 33 types of tumor patients and nine
GEO datasets. The expression profile data of different cancer patients revealed that ENG, FLT1, A2M, and PECAM1 were highly expressed in 13, 15, 15, and 16 out of 33 types of tumor tissues, respectively (Figure 3A and Figure S2A–C). Similarly, the expression
profile data of HCC patients in the GEO data sets found that ENG,
FLT1, and A2M were highly expressed in 7, 4, and 9 out of 9 different GEO data sets (GSE102097, GSE12128, GSE22058, GSE25097,
GSE36376. GSE46444, GSE57957, GSE76297, and GSE76427) (Figure 3B and Figure S2D and E). Subsequently, the expression level
of ENG mRNA was detected in HCC samples using the qRT-PCR
assay. The results indicated that ENG was significantly upregulated in adjacent tissues compared to tumor tissues (Figure 3C).
Furthermore, we further performed a western blotting assay in 25
paired human HCC samples to detect ENG expression and found
that ENG expression was consistent with ENG transcription level
(Figure 3D). These results indicated that the biomarkers of vascular ECs were highly expressed in adjacent tumor tissues of HCC.
ENG expression presents combating clinicopathological characteristics and a poor prognosis in HCC patients
The above result showed that ENG was highly expressed in
the adjacent tumor tissues. However, the role of ENG expression
was poorly elucidated in HCC patients. Here, we analyzed the relationship between the expression level of vascular ECs markers
and clinicopathological characteristics (that is, microvascular infiltration, pathological stage, pathological grade, age, gender, BMI,
AFP, and HBV). Although ENG expression was not correlated with
gender, age, BMI, and HBV infection (Figure 4), ENG expression
was positively correlated with tumor grade and microvascular infiltration. Furthermore, A2M expression was positively associated
with age (p=0.028) and AFP level (Figure S3, p=0.036), and PE-CAM1 expression was positively associated with tumor grade (Figure S4, p<0.05). Therefore, the vascular ECs markers ENG might
be a marker of microvascular infiltration in HCC. Subsequently,
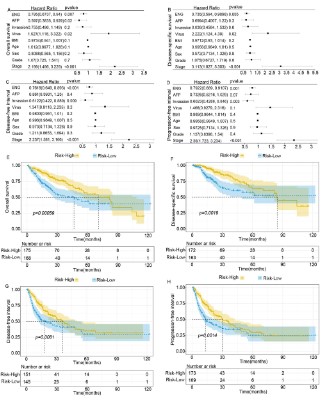
the multivariate analysis presented that ENG, together with microvascular invasion, AFP, virus, BMI, age, sex, tumor grade, and
tumor stage, was an independent risk factor for OS (HR= 0.795; P=
0.007), DSS (HR= 0.735; P= 0.005), DFI (HR= 0.7618; P<0.001) and
PFI (HR= 0.7922; P= 0.001, Figure 5A-D). Consistently, ENG was
weakly expressed in tumors and presented poor OS (P= 0.00059),
shorter time to DSS (P= 0.046), and PFI (P= 0.0014, Figure 5E-H).
On the contrary, the high expression of ENG in the adjacent areas
presented poor OS (P= 0.0017) and a shorter time for DSS (P=
0.046, Figure S5). These results suggested that ENG could be used
as an independent prognostic factor affecting the progression and
outcome of the disease.
ENG can promote the formation of HCC microvessels
As in the previous study, we found that ENG can be used not
only as a marker of the vascular endothelium, but also as an independent risk factor that affects the prognosis of HCC patients.
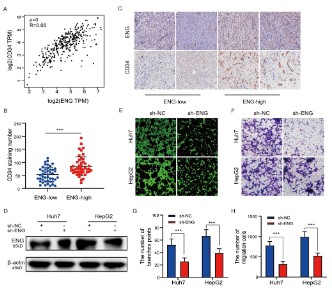
However, the effect of ENG on HCC tumor angiogenesis remains unclear. We first analyzed the correlation between ENG and the
vascular marker CD34 through the GEPIA database, and the results suggested that there was a significant positive correlation
between ENG and CD34 expression (Figure 6A). In addition, we
detected microvessel density (MVD) in tumor tissues of 65 HCC
patients, and MVD was counted by the number of blood vessels
stained with anti-CD34 antibody. The results showed that the
MVD of the high ENG expression group was significantly higher
than that of the low ENG expression group and the difference
was statistically significant (Figure 6B and C). On the basis of the
above results, we further explored the effects of ENG on the migration and tubulogenesis of vascular endothelial cells at the cellular level. We first transfected the lentivirus carrying ENG shRNA
into Huh7 and HepG2 cells, and the empty vector was used as
a control group (Figure 6D). After 72 hours of transfection, the
protein was extracted and detected by Western blot assay. The
results showed that the expression of ENG in Huh7 and HepG2
cells after interference with the expression of ENG was significantly lower than that in the blank control group. Subsequently,
we carried out transwell cell migration experiments and HUVECs
tubule formation experiments, and the results showed that the
number of tubule formation was significantly reduced (Figure 6E
and G), and the migration ability of HUVECs cells was weakened
(Figure 6F and H) after silencing the expression of ENG. Taken together, the above results suggested that ENG was involved in the
angiogenesis of HCC.
ENG promotes HCC microvascular formation by upregulating
the expression of COL1A1
To better understand the role of ENG in promoting HCC angiogenesis, we will further explore the mechanism by which
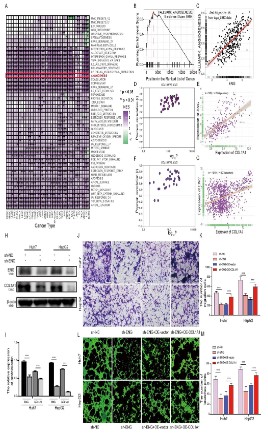
ENG regulates tumor angiogenesis in this chapter. We first performed an enrichment analysis of the pathway in patients with
different types of tumors and the results showed that almost all
types of cancer are involved in angiogenesis (Figure 7A and B).
Subsequently, we analyzed the correlation between ENG and angiogenesis, and the results also showed that ENG was positively
correlated with angiogenesis (Figure 7C). Studies have shown that
CoL1A1 is related to the occurrence and development of various
tumor diseases [24-26]. Therefore, we also analyzed the correlation between ENG and COL1A1 in the TCGA, LIHA, GTEx and CCLE
databases. The results indicated that ENG was positively correlated with COL1A1 (Figure 7D-G). To further verify the relationship
between ENG and COL1A1, we performed a Western blot assay
on a cell line with low expression of ENG and the results showed
that the expression of COL1A1 in Huh7 and HepG2 cells significantly decreased after interfering with the expression of ENG (Figure 7H and I). Based on the above results, we speculate that ENG
may be involved in HUVECs migration and tubule formation by regulating COL1A1 expression. Subsequently, we infected the Huh7
and HepG2 ENG low expression cell lines with the overexpressed
plasmid COL1A1 for 72 h and then immediately performed the
transwell cell migration assay and the HUVEC tubule formation
assay. The results showed that the migration capacity of HUVECs
was significantly reversed (Figure 7J and K), and the number of tubule formations was significantly recovered after up-regulation of
COL1A1 compared to the low expression group (Figure 7L and M).
ENG promotes immune cell infiltration
The above result showed that ENG was not only an independent prognostic factor and predicted a poor prognosis but also
could promoted angiogenesis in HCC. However, the role of ENG
in the tumor immune microenvironment (TIME) remains unclear.
Therefore, we performed bioinformatic analysis and found that
ENG expression in HCC may participate in the regulation of immune infiltrating cells and extracellular matrix (Figure S6). To clarify the complex mechanism of ENG in TIME, we calculated the
immune/stromal/estimated score and tumor purity using an estimate algorithm (Table S4). The result showed that ENG expression
was positively correlated with immune scores, stromal scores,
and Estimate scores, while negatively correlated with tumor purity (Figure S7A-D). These results suggested that ENG acted as an
essential part of the regulation of the tumor immune microenvironment of HCC. Thus, CIBERSORT was performed to quantify
the immune cells of patients with HCC (Table S5). Immune cells,
CD4 cells of main memory, CD8 cells of memory, natural killer
cells, and T cells of the natural killer were infiltrated in high-expressed patients with ENG (Figure 8A and B). Then, Spearman’s
correlation analysis was performed to establish the correlation
between ENG expression and the level of immune cell infiltration
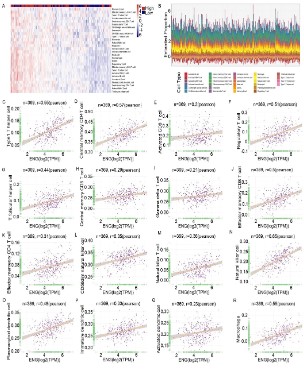
using quantitative data. As shown in Figure 8C-R, ENG expression
correlated positively with Type1 T helper cells (r = 0.55), central
memory CD4T cells (r = 0.57), activated CD8 T cells (r = 0.2), regulatory T cells (r = 0.51), T follicular helper cells (r = 0.44), central
memory CD8 T cells (r = 0.29), gamma delta T cells (r = 0.21), effector memory CD8 T cells (r = 0.5), effector memory CD4 T cells (r= 0.31), CD56dim natural killer cells (r = 0.35), natural killer T cells
(r = 0.36), natural killer cells (r = 0.65), plasmacytoid dendritic cells
(r = 0.48), immature dendritic cells (r = 0.33), activated dendritic
cells (r = 0.23), and macrophages (r = 0.55). In conclusion, the expression of ENG in HCC was involved in the regulation of immune
infiltrating cells.
ENG expression levels correlated with the antitumor efficacy
of sorafenib in HCC patients
As mentioned above, ENG is a marker of the vascular endothelium of HCC, and its high expression level can promote an increase in tumor tissue hematogenesis in HCC patients. Therefore,
antiangiogenic drugs can target patients with high expression of
ENG. We analyzed the correlation between ENG and angiogenesis
genes (targets of sorafenib: VEGFR, PDGFR, KIT, and VEGFA) in the
TCGA, LIHA, GTEx, and CCLE databases. The results indicated that
ENG was positively correlated with VEGFR, PDGFR, VEGFA, and
KIT, respectively (Figure S8). These results further illustrated that
sorafenib was a potential target drug in the treatment of high-expression ENG in HCC. To better understand the above findings
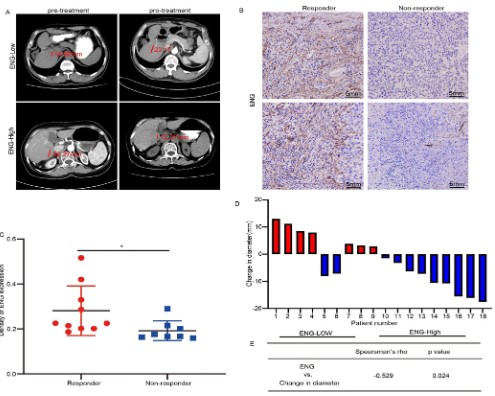
in cancer patients, 18 HCC patients treated with sorafenib, ten
responders and eight non-responders, were collected for ENG expression. Two representative cases of tumor diameter (red line)
to sorafenib therapy were shown in Figure 9A, and four representative cases of ENG were presented in Figure 9B. Importantly, ENG
was low expressed in patients without sorafenib response (Figure
9C and D), and ENG expression was positively correlated with the
change in diameter (Figure 9E). Together, these results suggested
that the property of sorafenib in tumor suppression was altered
for the expression of ENG.
Discussion
The high rate of recurrence, high metastasis, and low survival
rate are the main characteristics of HCC, which may be contributed by the highly vascularized features of HCC and the unique tumor microenvironment (TME) [27]. In recent years, angiogenesis
has been found to play an essential role in the progress of HCC.
The antitumor vascular therapy is widely applied in clinics. Unfortunately, due to the heterogeneity of tumor microvessels, the
effect of anti-angiogenesis therapy is limited [28]. Actually, bone
marrow-derived ECs [29], cancer stem cells-derived ECs [11], and
vascular channels-derived cancer cells [30] successfully formulate
neovascular in tumors. Here, multiple subpopulations with different profiles were identified in patients with HCC. In the process of
infiltration, tumor cells were retrodifferentiated to vascular markers with highly expressed endothelial-like cells. Instead of murine chromosomes, some ECs have typical human chromosomes
in tumors from mice transplanted with human glioblastoma [12].
Consistently, in our artwork, neovascular endothelial cells co-expressed the neovascular marker (CD31) and EGFP (exogenously
expressed in cultured Huh7 cells) or AFP (an HCC biomarker, Figure 2) in tumors of mice transplanted with Huh7 cells. These
results suggest that the ECs of angiogenesis are derived from tumor cells during tumor formation. Thus, neovascular oxygen and
nutrients are provided for rapidly formulated tumor tissues and
remove harmful metabolites [31].
ENG is expressed primarily in new capillary endothelial cells
at the edge of tumor tissue and is weakly expressed on the vascular ECs of normal tissues. More importantly, ENG promotes tumor cell proliferation, so ENG expression is known as an indicator
of dynamic observation of ECs proliferation [32,33]. In fact, our
findings support previous results that high ENG expression is a
significant enrichment in angiogenesis, extracellular matrix organization, and immune cell activation by GO and GSEA analysis (Figure S6). In addition, patients with high expression of ENG were
more likely to have lower overall survival. At the same time, the
univariate Cox regression analysis showed that ENG could be an
independent prognostic factor that affects OS, DSS, DFI, and PFI.
In this study, our team found that ENG can be used as a symbol of
vascular endothelial cells and can affect the prognosis of patients
with HCC. However, the current mechanism to regulate tumor
blood vessel production in HCC is unclear. We first confirmed that
ENG expression was positively related to CD34 expression through
GEPIA database analysis. At the same time, we also conducted an
IHC analysis of 65 cases of HCC samples, and the result prompted the ENG high expression group to have a higher microvascular density. Subsequently, we continued to explore the effects of
ENG on migration capacity and the formation of small tubes of
vascular endothelial cells. Therefore, the migration capacity of
HUVECs cells was reduced, and the number of small tubes was
significantly reduced after lowering ENG expression. These results
showed that ENG participated in the formation of HCC blood vessels. Therefore, ENG can be used as a potential treatment target
to inhibit the formation of new blood vessels of HCC. Finally, it is
possible to provide new ideas for HCC blood metastasis. To better
understand the role of ENG in promoting HCC angiogenesis, we
analyzed the correlation between ENG and angiogenesis, and the
results also showed that ENG expression was positively correlated with angiogenesis. Our team also found that ENG was positively correlated with COL1A1. COL1A1 is the composition of the
extracellular matrix, which is closely related to the growth, proliferation, and differentiation of cells[24]. Studies have reported
that COL1A1 is highly expressed in a variety of malignant tumors,
promoting tumor invasion and metastasis[26]. Therefore, we hypothesized that ENG might regulate COL1A1 expression and participate in vascular formation. Subsequently, we continued to verify
the relationship between ENG and COL1A1 at the cellular level,
and Western blot results showed that COL1A1 expression levels
obviously decreased after the downregulation of ENG expression.
To further explore whether ENG regulates COL1A1 expression
and can cause changes in the vascular production function, we
found that overexpression of COL1A1 could reverse the reduced
migration capacity and the reduced number of tubules in HUVECs
induced by the rescue assay. Therefore, we confirmed that ENG
is involved in HCC microvessel formation by regulating COL1A1.
Furthermore, according to reports in the literature [18,34,35], the
high expression of ENG in adjacent tissues is closely related to
recurrence after liver transplantation. To sum up the results, we
speculate that ENG can predict tumor recurrence after liver transplantation. It is recommended to actively reduce the level of ENG
after liver transplantation to inhibit the formation of new blood
vessels. Therefore, ENG is expected to be a prognostic indicator
of angiogenesis, recurrence, and metastasis in patients with HCC.
Tumor tissues are enriched with abundant tumor blood. To this
end, angiogenesis inhibitors, such as blocking pro-angiogenic factors, specifically inhibit tumor angiogenesis in anti-angiogenesis
therapy. Sorafenib, targeted therapy in the first-line treatment
of HCC, inhibits angiogenesis through the targeted inhibition of
VEGFR, PDGFR, KIT, and VEGFA in tumor suppression. Interestingly, our results showed that the expression level of ENG is positively correlated with the mRNA expression of the target genes
for sorafenib treatment, such as VEGFR, PDGFR, KIT, and VEGFA,
by analyzing the liver tissues, TCGA, LIHC, CCLE, GTEx, and TCGA
(Figure S8). It is widely accepted that the tumor suppressor effect
of sorafenib is widely believed to be achieved by regulating the
expression of specific target genes. Therefore, to illustrate that
sorafenib was a potential targeted drug to treat high-expression
ENG in HCC, we screened 18 patients treated with oral sorafenib
for HCC and then determined ENG expression in HCC by immunohistochemistry of pathological tissues and evaluated the therapeutic effect by CT. The results show that ENG expression levels
were correlated with the efficacy of sorafenib in patients with
HCC. Although the mechanism by which ENG directly regulates
the expression of sorafenib-specific target genes has not been effectively elucidated, the expression of ENG can indeed effectively
promote the antitumor effect of sorafenib in patients with HCC.
Therefore, our research is expected to provide new potential targets for tumor anti-angiogenesis therapy in clinics.
Immune cells are infiltrated into the tumor tissues. Unfortunately, immunomodulatory proteins and immune checkpoint
molecules suppressed the activation of immune cells infiltrated
by tumor tissue. Tumor immunotherapy reactivates immune cell
function by blocking overexpressed immunomodulatory proteins
in solid tumors, thereby inhibiting tumor progression of many
tumors. However, tumor immunotherapy failed to achieve a
stable and sustained antitumor effect. Our results showed that
the expression level of ENG was related to the degree of infiltration of immune cells in tumor tissues, suggesting that a large
number of immune cells are infiltrated in tumor tissues rich in
blood vessels. However, abnormally abundant tumor blood vessels lead to immunosuppression in the tumor microenvironment,
which dramatically interferes with the effect of immunotherapy.
Anti-angiogenesis therapy reprogrammed tumor blood vessels by
blocking pro-angiogenic factors, which effectively improved blood
perfusion, reduced hypoxia and acidosis, and relieved the tumor
microenvironment’s suppression of immune cells. Thus, a completely new antitumor strategy, combined application of targeted
therapy and immunotherapy, reconstructs the number and function of immune cells in tumor tissues, which is extremely attractive to patients.
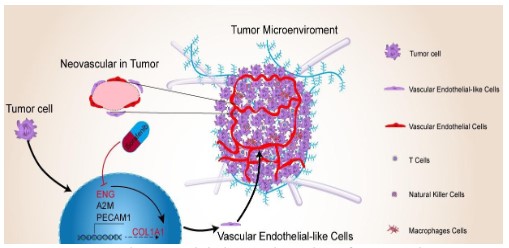
Conclusion
In general, this study has clarified four points. First, neovascular in HCC patients was formed by tumor-derived vascular endothelial cells. Second, the high expression of ENG in HCC adjacent tissues was significantly positively correlated with the level
of activated tumor immune cell infiltration, which indicated that
ENG had an immunomodulatory effect on tumor immunity. Third,
ENG promotes the formation of HCC microvessels by upregulating
the expression of COL1A1. Fourth, ENG promotes the antitumor
property of sorafenib in HCC. Finally, ENG can be used as a biomarker for the diagnosis, therapy, and prognosis of HCC (Figure
10). Although our study did not indicate the exact molecular mechanism by which ENG promotes the formation of new blood vessels and regulates the recruitment of tumor-specific immune cell
populations in tumors, the advantage of this research was that it
promotes the development of new precision-targeted immunotherapy research and prognostic and diagnostic biomarker.
Declarations
Data availability statement: All the original data and
information in this study are included in the article/Supplementary
Material. If you need more information, you can contact the
corresponding author.
Ethics statement: Research protocols involving humans
and animals have been reviewed and approved by the ethics
committee of Qingdao University Hospital. All participating
patients have signed written informed consent. All research
processes are carried out in accordance with the Declaration of
Helsinki.
Author contributions: TY contributed to designing and
supervising the study. CZ wrote the manuscript. YS and SS
collected and analyzed the data. YJ, XJ, and PJ raised the mouse
and checked the statistical method. QG and JD improved the
correction manuscript. The final submitted manuscript is
approved by all authors.
Funding: The capital health research and development of
special (2020-2-1152) and the National Science and Technology
Major Project (2018ZX10302205-005) provided this research
fund.
Acknowledgments: The authors acknowledge Hua Guo and
Shuo Han for their technical assistance. The authors also thank
Professor Dexi Chen (Beijing Institute of Hepatology, Beijing Youan
Hospital, Capital Medical University, Beijing, China) for sharing his
small animal in vivo laser confocal microscope and ultrasound
imaging system for these studies.
References
- Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for
advanced hepatocellular carcinoma: a focus on special subgroups.
Gut. 2021; 70: 204-214.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer
journal for clinicians. 2020; 70: 7-30.
- Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune
microenvironment and current immunotherapeutic strategies for
hepatocellular carcinoma, J Exp Clin Cancer Res. 2019; 38: 396.
- Oura K, Morishita A, Tani J, Masaki T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int J Mol Sci. 2021; 22
- Pinter M, Jain RK, Duda DG. The Current Landscape of Immune
Checkpoint Blockade in Hepatocellular Carcinoma: A Review.
JAMA oncology. 2021; 7: 113-123.
- Kalasekar SM, Garrido-Laguna I, Evason KJ. Immune Checkpoint
Inhibitors in Combinations for Hepatocellular Carcinoma, Hepatology (Baltimore, Md.). 2021; 2591-2593.
- Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS.
The Role of Angiogenesis in Hepatocellular Carcinoma, Clin Cancer
Res. 2019; 25: 912-920.
- Li H. Angiogenesis in the progression from liver fibrosis to cirrhosis
and hepatocelluar carcinoma. Expert Rev Gastroenterol Hepatol.
2021; 15: 217-233.
- Zhang HF, Gao X, Wang X, Chen X, Huang Y, et al. The mechanisms
of renin-angiotensin system in hepatocellular carcinoma: From
the perspective of liver fibrosis, HCC cell proliferation, metastasis
and angiogenesis, and corresponding protection measures, Biomedicine & pharmacotherapy Biomedecine & pharmacotherapie.
2021; 141: 111868.
- Bry M, Kivela R, Leppanen VM, Alitalo K. Vascular endothelial
growth factor-B in physiology and disease. Physiol Rev. 2014; 94:
779-794
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, et al.
Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010; 468: 824-828.
- Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, et al.
Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010; 468: 829-833.
- Pasterkamp G, Goumans MJ. The Microvasculature: The Next
Battlefield Where Transforming Growth Factor-beta and Endoglin
Draw Their Double-Edged Swords?, Arterioscler Thromb Vasc Biol.
2017; 37: 10-12.
- Harmsen MJ, Wong CFC, Mijatovic V, Griffioen AW, Groenman F,
et al. Role of angiogenesis in adenomyosis-associated abnormal
uterine bleeding and subfertility: a systematic review, Hum Reprod
Update. 2019; 647-671.
- Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications, FASEB journal: official
publication of the Federation of American Societies for Experimental Biology. 2003; 17: 984-992.
- Liu D, Kumar S, Ashworth J, Ali K, Fadel A, et al. CD105 (Endoglin):
A Potential Anticancer Therapeutic Inhibits Mitogenesis and Map
Kinase Pathway Activation. Anticancer research. 2021; 41: 1219-
1229.
- Kasprzak A, Adamek A. Role of Endoglin (CD105) in the Progression of Hepatocellular Carcinoma and Anti-Angiogenic Therapy, Int
J Mol Sci. 2018; 19
- Yang LY, Lu WQ, Huang GW, Wang W. Correlation between CD105
expression and postoperative recurrence and metastasis of hepatocellular carcinoma, BMC Cancer. 2006; 6: 110.
- Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma,
Anat Rec (Hoboken). 2008; 291: 721-734.
- Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pa-
thway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor
vasculature. Methods in enzymology. 2006; 407: 597-612.
- Mas VR, Maluf DG, Archer KJ, Yanek K, Kong X, et al. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma, Molecular medicine (Cam-
bridge, Mass.). 2009; 15: 85-94.
- Mínguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies, Current
opinion in gastroenterology. 2009; 25: 186-194
- Hendrix MJ, Seftor EA, Seftor RE, Chao JT, Chien DS, et al. Tumor
cell vascular mimicry: Novel targeting opportunity in melanoma.
Pharmacol Ther. 2016; 159: 83-92.
- Li J, Ding Y, Li A. Identification of COL1A1 and COL1A2 as candidate
prognostic factors in gastric cancer, World J Surg Oncol. 2016; 14:
297.
- Zhang Z, Wang Y, Zhang J, Zhong J, Yang R. COL1A1 promotes metastasis in colorectal cancer by regulating the WNT/PCP pathway,
Molecular medicine reports. 2018; 5037-5042.
- Ma HP, Chang HL, Bamodu QA, Yadav VK, Huang TY, et al. Collagen
1A1 (COL1A1) Is a Reliable Biomarker and Putative Therapeutic
Target for Hepatocellular Carcinogenesis and Metastasis. Cancers.
2019; 11.
- Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in
hepatocellular carcinoma: current status and therapeutic targets,
Semin Cancer Biol. 2011; 21: 35-43.
- Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, et al. Phase
II trial evaluating the clinical and biologic effects of bevacizumab in
unresectable hepatocellular carcinoma, J Clin Oncol. 2008; 2992-
2998.
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature
medicine. 2001; 1194-1201.
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, et al. Vascular channel formation by human melanoma cells in vivo and in
vitro: vasculogenic mimicry, The American journal of pathology.
1999; 739-752.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 646-674.
- Kimura H, Yasukawa T, Tabata Y, Ogura Y. Drug targeting to choroidal neovascularization. Advanced drug delivery reviews. 2001; 52:
79-91.
- Wang Y, Zhang XH, Guo P, Yan LN, He D. Tumor microvascular density detected by anti-CD105 and anti-CD34 in hepatocellular carcinoma patients and its predictive value of tumor recurrence after
liver transplantation. Sichuan da xue xue bao. Yi xue ban = Journal
of Sichuan University. Medical science edition 2010; 41: 818-821.
- Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, et al.
Vascular invasion and histopathologic grading determine outcome
after liver transplantation for hepatocellular carcinoma in cirrhosis, Hepatology (Baltimore, Md.). 2001; 33: 1080-1086.
- Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, et al.
Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation, Journal of gastrointestinal surgery : official journal of the
Society for Surgery of the Alimentary Tract. 2002; 224-232.