Introduction
With 11,48,515 new cases and 5,76,858 deaths worldwide in
the year 2020, colorectal cancer (CRC) represents the third most
commonly diagnosed cancer (after breast and lung cancers) and
the second leading cause of cancer death (after lung cancer) [1].
Increasing age, obesity, sedentary lifestyle, meat consumption, alcohol, and tobacco are considered the driving risk factors of CRC
[2]. According to the global cancer observatory 2020 report in Bulgaria, CRC ranks second, both in terms of cancer incidence (after
prostate cancer) and mortality (after lung cancer), with 4,648 new
CRC cases and 2,024 deaths reported in the year 2020.
Approximately, 5-years overall survival (OS) is 90% if detected
at an early stage. However, in case of metastatic CRC (mCRC), the
prognosis is poor, with a 5-year OS rate of only 14% [3,4]. Resection of metastases, especially in the liver, is currently the only
treatment that offers a chance of long-term OS [5]. The treatment
strategy when upfront resection is not possible, is to maximise
the chances of metastasis resectability with the help of systematic
therapies. In fit patients, the first-line treatment is usually a cytotoxic doublet combined with an epidermal growth factor receptor
(EGFR) inhibitor in case of RAS wild-type tumors of the left colon,
or a cytotoxic doublet or triplet (for suitable patients) combined
with bevacizumab for RAS mutant tumors or RAS wild-type tumors of the right colon or BRAF mutant tumors [6]. In second-line
setting, the chemotherapy backbone is usually changed and combined with an anti-angiogenic agent, regardless of RAS status [6].
Aflibercept is a recombinant fusion protein that blocks the activity of Vascular Endothelial Growth Factors (VEGF)-A, VEGF-B, and
Placental Growth Factor (PlGF) [7]. In the randomized, placebo-controlled phase III trial VELOUR, aflibercept in combination with
FOLFIRI significantly prolonged OS (hazard ratio [HR], 0.8; 95% CI,
0.7–0.9; p = 0.003) and progression-free survival (PFS) (HR, 0.8;
95% CI, 0.7–0.9; p < 0.0001) compared with FOLFIRI plus placebo
in patients with mCRC [8]. Moreover, despite the enrollment of
early progressors after adjuvant oxaliplatin-based chemotherapy,
known to have a poor prognosis, aflibercept plus FOLFIRI almost
doubled the response rate compared to FOLFIRI plus placebo
(19.8% versus 11.1%, p = 0.0001). Based on these data, aflibercept
in combination with FOLFIRI was approved in the United States in
the year 2012 and in Europe in the year 2013 for the treatment
of patients with mCRC, who are resistant to or progressed after
an oxaliplatin containing regimen. However, VELOUR trial did not
evaluate health-related quality of life (HRQoL), and only a minority of patients (30%) received prior targeted agents (bevacizumab
only, since EGFR inhibitors were not available at the time VELOUR
was recruiting).
The current prospective study evaluates the impact on HRQoL,
effectiveness, and safety of aflibercept plus FOLFIRI prescribed in
unselected Bulgarian patients with mCRC in current daily clinical
practice. The Functional Assessment of Cancer Therapy-Colorectal (FACT-C) questionnaire [9], specifically developed and validated to evaluate HRQoL of patients with CRC, was used in the current study.
Methods
Study design and patients
This was a multicentre, prospective, observational study conducted in 13 centres in Bulgaria. Patients with mCRC eligible
for treatment with aflibercept plus FOLFIRI as per physician
choice in daily clinical practice were enrolled in the study. Patients
participating to another clinical study and/or receiving aflibercept
through a compassionate use program were excluded.
The study was conducted in accordance with the principles
of the Declaration of Helsinki and Good Clinical Practice guidelines as well as national laws and regulations of Bulgaria. The
study was registered with the Bulgarian Drug Agency (НИП –
0007/09.05.2017) and approved by the ethics committee (КИ –
23/20.04.2017). Written informed consent was obtained from all
patients before participation.
Treatment
Patients were prescribed the recommended dose of aflibercept
(4 mg/kg of body weight), administered as an intravenous (iv) infusion over 1 hour, followed by the FOLFIRI regimen (irinotecan
180 mg/m2 iv plus leucovorin 400 mg/m² iv on day 1, followed
by an iv bolus of 5-FU 400 mg/m2 and a continuous iv infusion of
5-FU 2400 mg/m2 over 46 hours). The treatment cycle was repeated every 2 weeks. In order to reflect daily practice of physicians,
there was no specifications in the study protocol concerning the
number of cycles to be administered and potential dose reductions or delays.
Assessments
Since the study reflected daily clinical practice of participating
centres, no recommendations regarding duration of treatment, frequency of visits, and monitoring examinations (imaging, laboratory tests) were provided. Investigators were all experienced in treating patients with mCRC and managing anticancer chemotherapy.
The main assessment of interest was HRQoL using FACT-C
questionnaire [9]. Patients who participated in the study agreed
to fill in a validated translation of the FACT-C questionnaire at
baseline and every 2 cycles during aflibercept plus FOLFIRI treatment. FACT-C includes five domains: physical well-being (PWB; 7
items), social/family well-being (SWB; 7 items), emotional wellbeing (EWB; 6 items), functional well-being (FWB; 7 items), and
additional concerns (9 items). Domain scores were obtained as
the sum of all the individual item scores.
Each item was rated on a five-point Likert scale (Not at all =
0, A little bit = 1, Some-what = 2, Quite a bit = 3, Very much = 4)
reflecting patient feeling during the previous 7 days. Higher scores
meant better HRQoL.
Other assessments included PFS, tumor objective response
rate (ORR), disease control rate (DCR), OS, and safety. PFS is defined as the time from treatment initiation to the date of disease
progression or death. ORR is defined as the proportion of patients
with a complete response (CR) or partial response (PR) as best
response during therapy. DCR is defined as the proportion of patients with a CR, a PR, or stable disease (SD) as the best response
during therapy. OS is defined as the time from treatment initiation
to the date of death from any cause. Adverse events (AEs) occurring from the signature of the informed consent form until 30
days after the last administration of aflibercept plus FOLFIRI were
recorded, regardless of their relationship with aflibercept.
Collection of data was planned at baseline, 6 months (±3
months), and 12 months (±3 months) post-inclusion.
Statistical analysis
All analyses were descriptive and p values were exploratory,
therefore, no formal sample size calculation was performed. Approximately, 100 patients with mCRC were planned to be enrolled
in the study. HRQoL was evaluated in all patients with a baseline
and at least one post-baseline value. The safety population included all patients who received at least one cycle of aflibercept
plus FOLFIRI. Continuous data were presented as mean (SD). Categorical data were presented as absolute numbers with percentages. The Kaplan-Meier estimates (including curves) were computed and the 95% CI for the median PFS or OS was provided. Patients lost to follow-up were censored at the date of last contact.
When the date of last contact was missing, censoring was done at
the previously documented date of follow-up. No imputation of
missing values was performed. Statistical analysis was performed
using SPSS version 24.0.
Results
Patient characteristics
Between June 2017 and February 2020, 101 patients were enrolled and received at least one cycle of aflibercept plus FOLFIRI,
representing the safety population. Of them, 79 patients were
evaluable for HRQoL (i.e., one baseline and post-baseline value)
and 99 patients were evaluable for effectiveness. Patients’ clinical characteristics at inclusion are summarised in Table 1. Mean
age was 65.2 years, most patients (59.6%) were males, and 90.9%
of patients had an Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0 or 1. Most tumors were left-sided
(colon descendant 38.4%, rectum 36.4%) and RAS mutations were
detected in 56.6% of cases. At enrollment, metastases were mainly located in the liver (76.8%) or lung (43.4%). Overall, 91.9% had
a prior surgery of the primary tumor and 33.3% received prior adjuvant chemotherapy. The mean time elapsed from CRC diagnosis
to aflibercept initiation was 24 months. All patients had received
a prior oxaliplatin-based regimen and 78.7% had received a prior
targeted therapy (bevacizumab 54.5%, anti-EGFR 20.2%, both
anti-EGFR and bevacizumab 4.0%). Aflibercept plus FOLFIRI was
prescribed in second-line setting in 65.6%, in third-line in 26.3%,
and beyond third-line in 8.1% of cases. The median number of
cycles received was 6 (range: 1-24). At the end of the study, treatment was still ongoing in 4 patients and 95 patients had discontinued therapy, mainly due to disease progression (51.5%), patient
request (15.2%), or AEs (12.1%).
Health-related quality of life
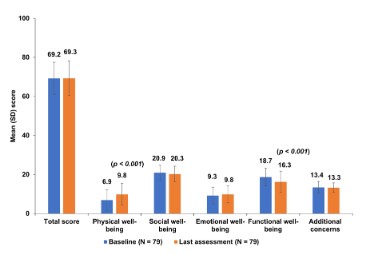
Overall, 79 patients completed the FACT-C questionnaire at baseline and at least once post-baseline. The mean total score was
69.2 at baseline and 69.3 at the last assessment during therapy (p
= 0.916). The mean PWB score improved significantly from 6.9 to
9.8 (p < 0.001) and the mean FWB score decreased significantly
from 18.7 to 16.3 (p < 0.001). No significant changes were observed in other dimensions (Figure 1). There was no relationship
between aflibercept therapy line and FACT-C average total score
and subscores (data not shown).
Effectiveness
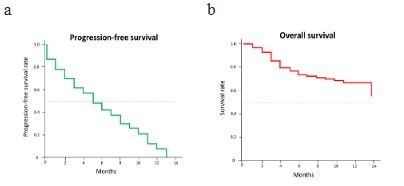
There were 71 progression or death events during the treatment period with a median PFS of 5 months (95% CI, 3.7–6.3). Objective tumor response was documented in 83 patients. Overall, 3
patients (3.6%) had a complete response, 8 patients (9.6%) had a
partial response, 39 patients (47.0%) had a stable disease, and 33
patients (39.8%) had a disease progression. The ORR was 13.3%
(11 out of 83) and the DCR was 60.2% (50 out of 83). Overall, 34
deaths occurred during the treatment period with a median OS
of 14 months (95% CI, 11.6–16.4). PFS and OS rates over time are
provided in Figure 2.
Subsequent therapies following aflibercept plus FOLFIRI were
documented for 41 out of 99 patients (41.4%): irinotecan-based
regimen (n = 14) associated with an anti-EGFR in 4 cases; oxaliplatin-based regimen (n = 13) associated with a targeted therapy
in 4 cases (anti-VEGF n = 2, anti-EGFR n = 2); capecitabine, n = 6;
regorafenib, n = 4; 5-FU, n = 3; and TAS 102, n = 1.
Table 1: Baseline clinical characteristics and treatment modalities
with aflibercept plus FOLFIRI.
| Characteristics |
ITT population (N = 99) |
| Age (years), mean (SD) |
65.2 (8.8) |
| Sex, % |
| Male |
59.6 |
| Female |
40.4 |
| Median BMI at enrollment, kg/m2(range) |
24.3 (16.2–35.8) |
| Performance (ECOG) status at visit 1, % |
| 0 |
30.3 |
| 1 |
60.6 |
| Median time from diagnosis to enrollment, months
(range) |
16 (2–106) |
| Primary site, % |
| Colon ascendens |
21.2 |
| Colon transversum |
7.1 |
| Colon descedens |
38.4 |
| Rectum |
36.4 |
| Metastatic sites, % |
| Liver |
76.8 |
| Lung |
43.4 |
| Lymph nodes |
21.2 |
| Peritoneum |
18.2 |
| Other |
16.2 |
| RAS status, % |
| Wild type |
27.3 |
| Mutant type |
56.6 |
| Unknown |
16.2 |
| Prior therapies, n (%) |
| Prior surgery |
91 (91.9) |
| Prior adjuvant chemotherapy |
33 (33.3) |
| Prior oxaliplatin-based regimen |
99 (100) |
| Prior targeted therapy |
| - Bevacizumab |
54 (54.5) |
| - Anti-EGF |
20 (20.2) |
| - Both (anti-EGFR and bevacizumab) |
4 (4.0) |
| - Unspecified |
1 (1.0) |
| Aflibercept plus FOLFIRI treatment modalities |
| mCRC therapy line, n (%) |
| - First line |
0 |
| - Second-line |
65 (65.6) |
| - Third-line |
26 (26.3) |
| - Beyond third-line |
8 (8.1) |
| Number of cycles |
| - Median (range) |
6 (1–24) |
BMI: Body Mass Index; ECOG: Eastern Cooperative Oncology Group;
EGFR: Epidermal Growth Factor Receptor; ITT: Intent-To-Treat.
Safety
The median duration of exposure to aflibercept plus FOLFIRI
was 84 days. During treatment, AEs of any grade were reported
by 52 patients (51.5%), mainly diarrhea (15.8%), fatigue (7.9%),
neutropenia and nausea (6.9% each), and stomatitis, weight loss
and hypertension (5.0% each). AEs were mild or moderate in most
cases (92.5%). None of the patients had embolism or reversible
posterior leukoencephalopathy syndrome. Serious AEs regardless
Table 2: Adverse events (safety population).
| Events |
Safety population (N = 101) |
| Any adverse event, n (%) |
52 (51.5) |
| Any serious adverse event, n (%) |
41 (40.6) |
| Common adverse events by decreasing order, n (%) |
| Diarrhea |
16 (15.8) |
| Fatigue |
8 (7.9) |
| Neutropenia |
7 (6.9) |
| Nausea |
7 (6.9) |
| Stomatitis |
5 (5.0) |
| Weight decreased |
5 (5.0) |
| Hypertension |
5 (5.0) |
| Decreased appetite |
4 (4.0) |
| Epistaxis |
4 (4.0) |
| Headache |
3 (3.0) |
| Thrombocytopenia |
2 (2.0) |
| Rectal haemorrhage |
2 (2.0) |
| Vomiting |
2 (2.0) |
| Blood creatinine increased |
2 (2.0) |
| Neuropathy peripheral |
2 (2.0) |
| Pruritus |
2 (2.0) |
| Anaemia |
2 (2.0) |
Percentages are based on N. Multiple occurrences of the same adverse
event in the same patient are counted only once. Events are presented
with ≥2% frequencies in the safety population.
of causality were reported by 41 (40.6%) patients and 27 patients
reported AEs leading to death (health status deterioration due to
disease progression, n = 23; hydronephrosis with multiorgan failure, n = 1; ischemic heart disease, n = 1; ileus, n = 1; and dehydration, n = 1). Listing of AEs through the study period is presented
in Table 2
Discussion
To the best of our knowledge, this is the first prospective observational study describing the impact of aflibercept plus FOLFIRI
on HRQoL using the FACT-C questionnaire in patients with mCRC.
Key messages may be summarized as follows: in this unselected
and heavily pretreated population reflecting daily clinical practice
in Bulgaria, aflibercept plus FOLFIRI showed no deleterious effect
on HRQoL assessed by FACT-C and retained its activity with a median PFS of 5 months, an ORR of 13.3%, a DCR of 60.2% and a
median OS of 14 months.
HRQoL has become increasingly important in patients with
mCRC since combinations of therapies used to prolong survival
may induce bothersome and long-lasting side effects which affect
patient daily lives. The FACT-C questionnaire has been specifically
developed to measure the impact of therapies on HRQoL in such
patients and is recognised as a valid and reliable tool which is sensitive to changes [9]. In our study, no significant changes in FACT-C
dimensions from baseline to last visit were observed, except for
the PWB, which was significantly improved and the FWB which was significantly reduced. These data support findings from other
observational studies which used different HRQoL instruments
such as European Organisation for Research and Treatment of
Cancer (EORTC) QLQ-C30, EORTC QLQ-CR29, EuroQol 5-Dimensions 3-Levels and also concluded that aflibercept plus FOLFIRI
has no deleterious effect on HRQoL [10-12]. We thus believe that
the data from our study would be helpful for the physicians in
their daily clinical practices.
Randomized placebo-controlled phase III trials provide evidence for the benefit/risk of therapies with the aim of getting
them registered but these trials enroll patients who satisfy stringent eligibility criteria and thus are not representative of patients
treated in daily clinical practice. This prospective observational
study thus appears complementary of the VELOUR phase III trial
since it enrolled patients who were older (mean age 65.2 versus
59.8 years) and less fit (ECOG-PS 2, 9.1% versus 2.2%) [8]. Compared to VELOUR, more patients received prior targeted agents,
either bevacizumab (58.6% versus 30.4%) or anti-EGFR (24.2%
versus 0%). Aflibercept plus FOLFIRI was also prescribed at a more
advanced disease stage since 34.4% received the regimen in third-line setting or beyond versus none in VELOUR. In this unselected
and heavily pretreated population, the activity of aflibercept plus
FOLFIRI was almost comparable to VELOUR trial in terms of PFS
(5 months versus 6.9 months), ORR (13.3% versus 19.8%) and OS
(14 months versus 13.5 months) [8]. The ORR in our study also
appeared higher than that observed in the ML18147 trial (6%
with bevacizumab continuation plus chemotherapy versus 4%
with chemotherapy alone in second-line) [13], possibly reflecting
the fact that aflibercept is the unique anti-angiogenic blocking the
PlGF, a known biomarker associated with resistance to bevacizumab [14].
In this unselected population reflecting daily practice of physicians no new safety signals were observed. The most frequently
reported AEs were diarrhea, fatigue, neutropenia, nausea, stomatitis, and hypertension, which are consistent with the known
safety profile of aflibercept plus FOLFIRI [8,10-12]. No unexpected
AEs were reported.
Limitations
This study has some limitations. First, this prospective observational study evaluated the daily practice of physicians, enrolled
patients who were unselected, and more heterogeneous than in
randomized clinical trials. Second, the timing of follow-up visits
and tumor assessments were not prespecified, and there was
no central review of imaging. These factors may have affected
the evaluation of ORR and PFS. However, no major differences
compared to the VELOUR trial were observed, in terms of tumor
response, PFS, and OS [8]. The results of laboratory tests were
not recorded and angiogenic biomarkers (PGF, VEGF-A) were not
analyzed, precluding a comparison with the VELOUR trial. Lastly,
the safety profile should be interpreted with caution due to the
possible underreporting in a real-world setting.
Conclusion
This prospective observational study evaluated the use of aflibercept plus FOLFIRI in the current mCRC treatment landscape in
Bulgaria. Results suggest that aflibercept plus FOLFIRI has no deleterious impact on HRQoL (FACT-C questionnaire) and retains its activity in unselected and heavily pretreated patients in routine
clinical practice. No new safety signals were observed. Aflibercept
plus FOLFIRI may thus represent an appropriate treatment option
in this setting.
Declarations
Acknowledgments: The authors are grateful to all study participants and would like to thank all the staff and investigators who
participated in the data collection for the study (participating physicians are listed in the supplement). The authors thank Krisztina
Hrács, PhD and János Fekete, PhD, at Adware Research Development and Consulting Ltd. for performing the data analyses of this
manuscript. Coordination of the development of this manuscript,
facilitation of authors discussion, and critical review was provided
by Helena Andersson, PhD, at Sanofi. The authors acknowledged
medical writing and editorial assistance provided by Niladri Maity,
PhD, at Sanofi. The authors were responsible for all content and
editorial decisions, and received no honoraria related to the development of this publication.
Conflict of interest: Dr Krassimir Koynov received fees for
consulting or advisory services from MSD, Eli Lilly, Pfizer, Roche,
BMS, Astra Zeneca, Takeda, Janssen, and Servier; has received
honoraria from MSD, Eli Lilly, Pfizer, Roche, BMS, Astra Zeneca,
Janssen, Merck, Novartis, Boehringer Ingelheim, Takeda, Gedeon
Richter, Zentiva, Viatris, Servier, Amgen, Sanofi, Astelas, and
Bayer; has received travel grants, accommodations, or other expenses from MSD, Roche, Pfizer, Astelas, Boehringer Ingelheim,
Bayer, Sanofi, Merck, Amgen, and Astra Zeneca. Dr Manol Slavov
received fees for consulting and advisory services from MSD, Servier, and BMS; has received honoraria from MERC, Eli Lilly, Servier,
and Amgen. Dr Christine GeffriaudRicouard is an employee of Sanofi and may hold shares and/or stock options in the company.
Dr Ivan Bivolarski declared no conflict of interest.
Funding: The study was funded by Sanofi. Statistical analysis
was performed by Adware Research Development and Consulting
Ltd., which was contracted by Sanofi.
Data sharing statement: Qualified researchers may request access to patient level data and related study documents including
the clinical study report, study protocol with any amendments,
blank case report form, statistical analysis plan, and dataset
specifications. Patient level data will be anonymised, and study
documents will be redacted to protect the privacy of our study
participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at:
https://www.vivli.org/.
Abbreviations: AEs: Adverse Events; CRC: Colorectal Cancer;
DCR: Disease Control Rate; ECOG-PS: Eastern Cooperative Oncology Group performance status; EGFR: Epidermal Growth Factor
Receptor; EWB: Emotional Well-Being; FACT-C: Functional Assessment of Cancer Therapy-Colorectal; FWB: Functional Well-Being;
HR: Hazard Ratio; HRQoL: Health-related Quality of Life; iv: intravenous; mCRC: Metastatic CRC; ORR: Objective Response Rate;
OS: overall survival; PFS: Progression-Free Survival; PlGF: Placental Growth Factor; PWB: Physical Well-Being; SWB: Social/Family
Well-Being; VEGF: Vascular Endothelial Growth Factors.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al.
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence
and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71: 209-249.
- Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence.
Lancet Oncol. 2017; 18: e457-e71.
- Chambers AC, Dixon SW, White P, Williams AC, Thomas MG, et al.
Demographic trends in the incidence of young-onset colorectal
cancer: a populationbased study. Br J Surg. 2020; 107: 595-605.
- Wang J, Li S, Liu Y, Zhang C, Li H, et al. Metastatic patterns and
survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 2020; 9: 361-373.
- Osterlund P, Salminen T, Soveri LM, Kallio R, Kellokumpu I, et al.
Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic
colorectal cancer patients (RAXO): A nationwide prospective intervention study. Lancet Reg Health Eur. 2021; 3: 100049.
- Cutsem EV, Cervantes A, Nordlinger B, Arnold D. ESMO Guidelines
Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
2014; 25: iii1-9.
- Syed YY, McKeage K. Aflibercept: A Review in Metastatic Colorectal
Cancer. Drugs. 2015; 75: 1435-1445.
- Cutsem EV, Tabernero J, Lakomy R, Prenen H, Prausová J, et al.
Addition of aflibercept to fluorouracil, leucovorin, and irinotecan
improves survival in a phase III randomized trial in patients with
metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012; 30: 3499-506.
- Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;
8: 181-195.
- Riechelmann RP, Srimuninnimit V, Bordonaro R, Kavan P, Bartolomeo MD, et al. Aflibercept Plus FOLFIRI for Second-line Treatment of Metastatic Colorectal Cancer: Observations from the Global Aflibercept Safety and Health-Related Qualityof-Life Program
(ASQoP). Clin Colorectal Cancer. 2019; 18: 183-1891.
- Hofheinz R-D, Anchisi S, Grünberger B, Derigs HG, Zahn M-O, et al.
Real-World Evaluation of Quality of Life, Effectiveness, and Safety
of Aflibercept Plus FOLFIRI in Patients with Metastatic Colorectal
Cancer: The Prospective QoLiTrap Study. Cancers. 2022; 14: 3522.
- Pastorino A, Di Bartolomeo M, Maiello E, Iaffaioli V, Ciuffreda L, et
al. Aflibercept Plus FOLFIRI in the Real-life Setting: Safety and Quality of Life Data From the Italian Patient Cohort of the Aflibercept
Safety and Quality-of-Life Program Study. Clin Colorectal Cancer.
2018; 17: e457-70.
- Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol.
2013; 14: 29-37.
- Cutsem EV, Paccard C, Chiron M, Tabernero J. Impact of Prior Bevacizumab Treatment on VEGF-A and PlGF Levels and Outcome
Following Second-Line Aflibercept Treatment: Biomarker Post
Hoc Analysis of the VELOUR Trial. Clin Cancer Res. 2020; 26: 717-
725.