Introduction
Gastrointestinal Stromal Tumors (GISTs) are the most common
mesenchymal tumors of the alimentary tract; they arise from the
interstitial cells of Cajal [1], pacemaker cells interposed between
the smooth muscle cells of the digestive tract and intramural neurons and characterized by the over expression of tyrosine kinase
receptor C-Kit [2], (CD117), a type III tyrosine kinase receptor for
stem cell growth factor, were found to be the source of GISTs [3].
GISTs can associate on other mutations [5,6]: about 5% of cases
are part of family genetic syndromes. The most common site of involvement is the stomach (56%), which is characterised by a better prognosis, followed by the small intestine (32%) and then the
colon and rectum (6%) [1], although they can occur at any level of
the digestive tract and occasionally in the omentum, mesentery
and peritoneum. Most cases of GISTs are sporadic. The correct
diagnosis of GIST is determined by histopathological examination
and immunohistochemistry [7].
They are frequently asymptomatic and may be detected incidentally on imaging studies for other indications. In particular
small size GISTs are usually asymptomatic and are diagnosed incidentally on radiological imaging for a different purpose or during
an endoscopic exploration, or during surgery. Large tumors may
cause abdominal distention, compression of the gastrointestinal
tract (for example GISTs with exophytic growth) or obstruction
of the gastrointestinal lumen (tumors with endophytic growth).
Symptomatic GISTs often make patient suffer from early satiety,
vague abdominal discomfort, nausea, upper GI bleeding, producing anemia and melena. Clinical manifestations are based on size
and tumor location. In the absence of complications, such as upper digestive hemorrhage/hemoperitoneum, intestinal obstruction, tumor perforation, or obstructive jaundice, the symptoms
are nonspecific (anemia, early satiety, swelling, abdominal pain)
[8]. The most common manifestation is gastrointestinal bleeding,
accompanied by anemia, hematemesis or melena. Dysphagia is
the main symptom encountered in esophageal GISTs. Furthermore, metastases can occur in advanced stages.
Suspected GISTs are usually evaluated with computed tomography (CT) and magnetic resonance imaging (MRI) scans; CT scan
remains the preferred initial imaging method used for staging the
disease. The lesion appear as hypervascular, heterogenous, and
enhancing masses, which displace rather than invade adjacent organs., They appear as smooth submucosal elevations on upper GI
endoscopy. Although endoscopy is useful to characterize and locate the lesion, biopsies rarely obtain adequate tissue to confirm
the diagnosis. Endoscopic ultrasound can confirm the origin of
the tumor from the submucosal layer and allow for image-guided,
deep sampling. In GISTs found in specific locations (e.g., the rectum) or in evaluating the anatomical extension of surgery, MRI
may be a better imaging option [9].
The survival rate of patients with GIST depends on multiple
factors: recurrence after treatment, risk category, GIST stage, or
treatment applied. Thus, patients with localized GISTs have a 5-year life expectancy of 93%, while patients with locally advanced
GISTs have a 5-year survival rate of 80%, and those with metastatic GISTs of 55% [4]. Risk stratification of GISTs attempts to define the risk of a poor outcome and to identify patients who may
benefit from adjuvant therapy.
Clinically, the classification scores by Fletcher et al. And Miettinen and Lasota are the most widely accepted ones. Fletcher et
al. classify the risk of aggressive evolution in four classes, depending on mitotic rate and tumor size:
• Very low risk: tumoral size <2 cm; mitotic count <5/50 high-power field;
• Low risk: tumoral size 2–5 cm; mitotic count <5/50 high-power field;
• Intermediate risk: tumoral size <5 cm and mitotic count
6–10/50 high-power field, or tumoral size 5–10 cm and mitotic count <5/50 high-power field;
• High risk: tumoral size >5 cm and mitotic count >5/50 high-power field, or tumoral size >10 cm and any mitotic rate, or
any tumoral size and mitotic rate >10/50 high-power field
[10].
Compared to GISTs that are localized in the small intestine or
rectum, gastric localization of GISTs is associated with a better
prognosis [11].
Surgical treatment
The role of minimal invasive surgery has been growing in recent years, while traditionally, open surgery has been advocated
for GISTs, expecially for fear of peritoneal seeding. The use of a
transgastric approach avoids the potential complication of luminal stenosis following a wedge resection of a tumor close to the
cardia. Local invasion is uncommon so that lymphadenectomies
are rarely required: a wide local resection is usually curative. Irrespective of tumor size, a laparoscopic approach can be considered
as the first line in uncomplicated GISTs [1]. Laparoscopic approach
is safe and feasible for Gastric GIST both in urgent and elective
settings. Laparoscopy shows a recurrence rate similar to open
surgery when radical resection are performed, even for lesions
greater than 5 cm. It is important to take in consideration the very
surgical team experience, one of the most important factors reducing the incidence of operative complications with better longterm outcomes, both oncological and postoperative [2]. While
traditionally, open surgery has been advocated for GISTs, for fear
of peritoneal seeding, the role of minimal access surgery has been
growing in recent years. The use of a transgastric approach avoids
the potential complication of luminal stenosis following a wedge
resection of a tumor close to the cardia. Because lymphadenectomies are rarely required and local invasion is uncommon, a wide
local resection is usually curative. Thus, a laparoscopic approach
can be considered as the first line in uncomplicated GISTs, irrespective of tumor size [1]. A wide, local resection with a 1 to 2
cm margin may be considered adequate. Additionally, a stapled
wedge gastrectomy would lead to a loss of significant uninvolved
gastric wall with the potential for significant luminal compromise.
It has also been seen that the margin of resection does not significantly affect the outcome, with similar recurrence-free survival in
patients who had R0 or R1 resections.
One potential shortcoming of transgastric approach is com-
promising the vascularity of the greater curvature, which can lie
between two longitudinal gastric incisions. It is usefull to confirm
adequate vascularity of the greater curvature in patients with the
use of indocyanine green (ICG) fluorescence angiography.
Histopatology
The section surface may be homogeneous, seen mostly in
small-size GISTs, or heterogeneous, with areas of hemorrhage
and necrosis in larger tumors. In small tumors, the coating mucosa remains unchanged (appearing normal), but in large, more
aggressive tumors, it may ulcerate. There are three main types of
GISTs: spindle cell type (70%), epithelioid type (20%) and mixed
type (10%) [3]. These tumors may range from small, benign lesions to large, hemorrhagic and necrotic masses with metastases.
Macroscopically, GISTs are well-defined, firm consistency, white in
color and not encapsulated [7].
Case report
Here we report a case of a 80-year-old female patient who was
being evaluated for weakness, anemia, and hematemesis.
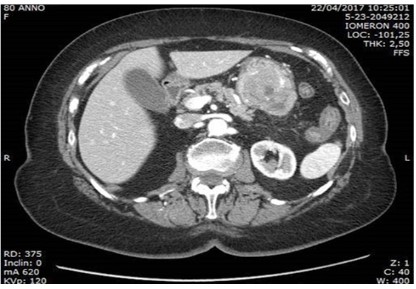
An abdominal computed tomography (CT) confirmed the presence of a voluminous ETP-like lesion, intensely capturing contrast
medium, of about 6 cm in maximum diameter, apparently originating from the submucosa of the gastric body, with extra-parietal
extension at the level of the great gastric curve, and with erosion
of the mucosa on the internal side. Hyperdense material is detected inside the gastric lumen as from bleeding in progress and in the
arterial phase the lesion appears vascularized by a large arterial
feeder starting from the A. gastric. No significant lymphadenomegaly or secondary lesions affecting the abdominal parenchymatous
organs are noted. Diverticulosis of the sigmoid colon. Not free air.
No versmental flaps in the abdomen. Adjust the size and course
of the great abdominal vessels (ATS of the abdominal aorta). Non-pleuro-pericardial effusion.” The ASA score estimated was III.
A bleeding ulcerated lesion of the gastric body was endoscopically found at the level of the posterior wall which couldn’t be
controlled endoscopically.
Operative steps
Laparoscopic access in the peri-umbilical site and under vision
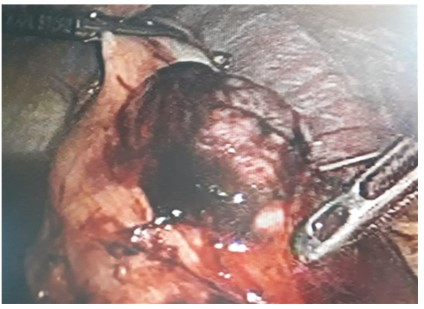
of another 3 trocars in the usual sites; negative exploration for hepatic and / or peritoneal repeats; opening of the gastrocolic ligament with Ultracision and access to the retrocavity of the epiploons, with a finding of neoformation on the posterior gastric wall
(Figure 2), with a prevalent exophytic development, of about 6 cm
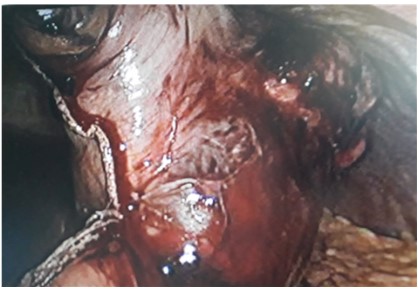
of max diameter, facing great curvature, below the fundus. Further mobilization of the stomach: we proceed to sleeve gastrectomy including the aforementioned lesion, with endo gia (4 refills
of 60) (Figure 3). Some hemostatic points on the cut, intracorporeal. Positioning of SNGs; washes, n. 1 drain; layered synthesis of
10 mm breccias.
The operative time was 120 minutes, and there was not significant blood loss. Postoperatively, the patient recovered well and
was discharged by the eight postoperative day. To date the patient
is in good health and disease free; control EGDS one year after
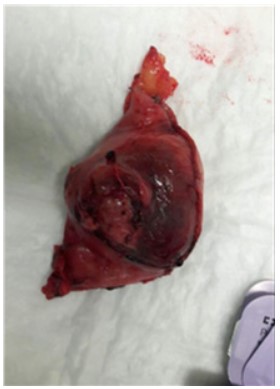
surgery reported normal gastroresection outcomes. Anatomopathological examination reported at the level of the submucosal tunic, a neoformation of the largest diameter (estimated after fixation) of 5.5 cm, of a yellowish-white color, with hemorrhagic
areas, duraelastic consistency, fasciculated appearance and well
demarcated margins is identified. The neoformation does not ulcerate the mucous membrane, it is 0.5 cm from the surgical resection margin and is close to the serous cassock (Figure 4). At the
microscopical examination, a mesenchymal neoplasm consisting
mainly of epithelioid cells, which develops in the context of the
gastric wall (from the submucosal to the subserosal cassock) was
found, with the presence of hemorrhagic areas. Absence of necrosis, calcifications, cellular pleomorphism. Mitotic index: low (2
mitoses / 5 mm2); proliferative activity (evaluated with Ki-67%):
low (2%). Surgical resection margin and deep margin unharmed.
Positivity for vimentin, C-KIT (CD117), DOG-1 (ANO1), CD34 and
negativity for desmin, smooth muscle actin, S100 were detected.
Discussion
Gastric GISTs with dimensions ≤4 cm can benefit from safe endoscopic resections. Gastric GISTs with dimensions >4 cm have a
risk of recurrence or even metastasis, and may require adjuvant
therapy with tyrosine kinase inhibitors (TKIs), or even the combination of an endoscopic and surgical technique [12].
Complete surgical resection remains the mainstay of treatment for non-metastatic GIST. It is the very only potentially curative therapy. It is now understood that all GISTs have some malignant potential, while traditionally GISTs were thought to exist on a
spectrum from benign to malignant. Mitotic index and tumor size
and are the two principal attributes, which help stratify malignant
potential of the tumor. These tumors are good candidates for
minimal access surgery, laparoscopic surgery was only considered
for smaller GISTs, up until a few years ago. Studies have shown
that as long as the aforementioned oncological principles are followed, laparoscopic surgery has better short-term outcomes in
the view of decreased shorter hospital stay and blood loss. A recent meta-analysis showed that long-term outcomes were found
to be equivalent to open surgery, even for larger GISTs.
Follow up
Although the risk of recurrence is not zero, patients at very
low risk may not require postoperative follow-up. In low-risk patients, a CT scan examination is recommended every 6 months
for 5 years. Intermediate–high risk patients require postoperative follow-up by CT examination at 3 months in the first 3 years,
then at 6 months for 5 years, then annually. There is a consensus
that abdominal ultrasonography can replace CT evaluation once a
year. In patients that are undergoing TKI therapy, PET/CT is sensitive for assessing treatment response, tumor recurrence or treatment resistance [13].
Conclusion
So, minimally invasive surgery can be considered the first approach for uncomplicated cases, irrespective of their size. The
combined approach both endoscopic and laparoscopic may allow
a better exposure of the tumour which ensure a radical resection
and has shown to be an effective technique [2]. Appropriate patient selection and advanced laparoscopic skills are critical to ensure that oncologic principles in the management of GIST of the
stomach are not compromised.
References
- Arora E, Gala J, Nanavati A, Patil A, Bhandarwar A. Laparoscopic
Transgastric Resection of a Large Gastric GIST: A Case Report and
Review of Literature. Surg J (N Y). 2021; 7: e337-e341.
- Di Buono G, Maienza E, Buscemi S, Bonventre G, Romano G, et
al. Combined endo-laparoscopic treatment of large gastrointestinal stromal tumor of the stomach: Report of a case and literature
review. Int J Surg Case Rep. 2020;77S: S79-S84.
- Gheorghe G, Bacalbasa N, Ceobanu G, Ilie M, Enache V, et al. Gastrointestinal Stromal Tumors-A Mini Review. J Pers Med. 2021; 11:
694.
- American Society of Clinical Oncology.
- Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016; 19: 3-14.
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013; 382: 973-983.
- Fulop E, Marcu S, Milutin D, Borda A. Gastrointestinal stromal tumors: Review on morphology, diagnosis and management. Rom J
Morphol Embryol. 2009; 50: 319-326.
- El-Menyar A, Mekkodathil A, Al-Thani H. Diagnosis and management of gastrointestinal stromal tumors: An up-to-date literature
review. J Can Res. 2017; 13: 889-900
- Scarpa M, Bertin M, Ruffolo C, Polese L, D’Amico DF, et al. A systematic review on the clinical diagnosis of gastrointestinal stromal
tumors. J Surg Oncol. 2008; 98: 384-392
- Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach.
Int J Surg Pathol. 2002; 10: 81-89.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology
and prognosis at different sites. Semin Diagn Pathol. 2006; 23: 70-
83.
- Zhang Y, Mao XL, Zhou XB, Yang H, Zhu LH, et al. Long-term outcomes of endoscopic resection for small (≤4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer. World J Gastroenterol. 2018; 24: 3030-3037.
- Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, et al. Gastrointestinal stromal tumours: ESMO–EURACAN Clinical Practice
Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol.
2018; 29.