Introduction
Vasculogenic mimicry (VM), the phenomenon by which tumour cells mimic ECs and form vascular channels themselves in
the first reported in 1999 [1-3], It refers to the plasticity of invasive cancer cells to form new vascular networks, thus contributing
to the perfusion of rapidly growing tumors, delivery of fluid from
leaking vessels, and/or connection with constitutional endothelial
layer vessels [4]. Over the next 20 years, VM has been reported
in a variety of malignancies, including melanoma, glioblastoma,
osteosarcoma, and hepatocellular carcinoma, as well as breast, lung, gastric, colorectal, and prostate cancers. In patients with
malignancies such as breast, colorectal, prostate, liver, lung, ovarian, gastric, and bladder cancers, VM is associated with high
tumor grade, invasion, metastasis, and poor prognosis [5].VM is
considered an important factor in the poor anti-tumor angiogenesis effect gradually becoming a hot research topic. However, the
mechanism of how tumor cells build into microvascular channels
is not clear.
Some studies have shown that a hypoxic and acidic microenvironment that strongly favours VM in xenografts [5,6]. Tumor activated platelets also possibleinduce vascular mimicry in me-
senchymal stem cells and aid metastasis [7]. A study showed that
microbial metabolite deoxycholic acid promotes vasculogenic mimicry formation in intestinal carcinogenesis [8]. All these factors
are involved in the formation of VM. Hypoxic and acidic microenvironment is closely associated with epithelial mesenchymal transition (EMT) [9-11]. EMT is a process in which epithelial cells, under the action of some factors, lose their cell polarity, lose their tight intercellular connections and adhesion connections, and gain
the ability of infiltration and migration, becoming mesenchymal
cells with morphology and characteristics [12]. Whether EMT is a
VM driver raises concerns. We speculate that EMT enables tumor
cells to acquire tentacles and invasive ability, which may be one of
the driving factors for their formation of VM. A small amount of
literature mentions the association of EMT with angiogenic mimic
formation in some tumors, such as gastric cancer [13] and melanoma [14]. However, the exact relationship between EMT and VM
still needs to be further clarified.
Nasopharyngeal carcinoma (NPC) is a malignant tumor of nasopharynx with high affection in Southeast Asia [15]. In previous
studies, we have shown that Foxq1 significantly promotes Vasculogenic mimicry (VM) formation, tumor growth, and metastasis
and is effectively inhibited by EGFR inhibitors [16]. In the text we
suggest that EGFR promotes the formation of VM. Whether EMT
is an EGFR-regulated VM is worth further investigation.
Materials and methods
Clinical samples
60 cases of nasopharyngeal carcinoma tissue samples were
collected from the Southern Hospital of Southern Medical University. The research subjects selected nasopharyngeal carcinoma
patients with pathologically confirmed nasopharyngeal carcinoma from 2007 to 2019. Detailed pathological, clinical data and
survival time of all NPC patients were collected through outpatient and telephone follow-up. Each patient signed an informed
consent form. The TNM grading is based on the definition of the
UICC American Joint Committee on Cancer Staging Criteria, 7th
edition. The employment of these tissue samples was approved
by the Ethics Committee of Nanfang Hospital, Southern Medical
University.
Immunohistochemical and CD31-PAS dual staining
The tissue was fixed with formalin, embedded in paraffin, and
sliced at a thickness of 4 mm. After collection, the tissue was fixed
with 4% paraformaldehyde at 4°C overnight. Antigen blocking
was performed using 10% goat serum (AR0009, Boster, China).
Anti-e-cadherin ((24E10) Rabbit mAb #3195, CST), anti-cd31 ((PE-CAM-1) (D8V9E) XP Rabbit mAb #77699, CST), anti-Ve-cadherin
((D87F2) XP Rabbit mAb #2500, CST) and antibodies against vimentin ((D21H3) XP Rabbit mAb #5741, CST) were incubated
overnight at 4°C. DAB system (ZLI-9017, Zsbio, China) was used
to detect staining. Vasculogenic mimicry structures were detected using PAS staining kit (G1281, Solarbio, China) and anti-cd31
((PECAM-1) (D8V9E) XP Rabbit mAb #77699, CST). The number of
positive cells was obtained from 5 randomly selected fields and
400x magnification.
Immunohistochemical score
Immunohistochemical scoring criteria: comprehensive score =
staining intensity × positive area. Staining intensity score: strong
positive (3 points), positive (2 points), weak positive (1 point),
negative (0 points). The proportion of positive (including strong
positive) regions: 100%-76% (4 points), 75%-51% (3 points), 50%-26% (2 points), 0-25% (1 point). The above scores were averaged
by two pathologists independently.
Cell culture
All nasopharyngeal carcinoma cells were obtained from the
Cancer Research Center of Southern Medical University, and
were cultured in RPMI-1640 medium (Thermo Fisher Scientific
Corporation PM15101) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific Corporation 10270-106), 100 u/
ml penicillin (15140-122, Thermo Fisher Scientific, USA), 100 mg/
ml streptomycin (15140-122, Thermo Fisher Scientific, USA), and
humidified in 5% CO2 The environment was maintained at 37°C.
Three-dimensional culture
24-well plates coated with 100 μL Matrigel (354230, BD
Biosciences, USA) reduced growth factor for each well, incubated
at 37OC for 1 h, Take 500 μL medium containing 10% FBS (1*105
cells), spread it on the gel surface, and incubate at 37OC for 24
h. Each group provides three holes. The cells were then photographed under an inverted microscope (IX71, OLYMPUS, Japan).
ImageJ calculates the average number of tubular structures.
RNA isolation, reverse transcription, and quantitative Real-time PCR
Total RNA was extracted from samples using RNA iso Plus
(R401-01, Vazyme, China) and reverse transcribed using HiScipt
III RT SuperMix for Quantitative Real-time PCR (+gDNA wiper)
(R323-01, Vazyme, China) as cDNA. Quantitative reverse transcription PCR (qRT-PCR) was performed on ABI QuantStudio5
system using ChamQ SYBR qRT-PCR Master Mix (Low ROX master mix) (Q331-02, Vazyme, China). GAPDH served as an mRNA
endogenous control. All samples were normalized to an internal
control and relative expression levels were calculated by using relative quantification.
Western blot
The proteins extracted from samples were assayed using lysis
buffer (P0013B, Beyotime, China) containing protease inhibitor
cocktail (HY-K0010, MCE, USA) using radioimmunoprecipitation.
Proteins were solubilized in SDS loading buffer (FD006, Fdbio,
China), and the lysates were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (IPVH00010, Millipore, USA).
Anti-E-Cadherin (24E10, CST, USA), Vimentin (D21H3, CST, USA),
VE-Cadherin (D87F2, CST, USA), N-Cadherin (D4R1H, CST, USA),
β-Catenin (D10A8, CST, USA) or GAPDH (D16H11, CST, USA) polyclonal antibodies were incubated at 4°C overnight at a dilution of
1:1000, and then incubated with species-specific enzyme-labeled
secondary antibodies (1:5000 dilution) for 2 h at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence (WBKLS0100, Millipore, USA).
Statistical analyses
Statistical analysis was performed using SPSS 25.0 software.
All data are from at least three independent experiments. Unless
otherwise stated, data are presented as SEM means. A p-value <0.05 was considered statistically significant.
Results
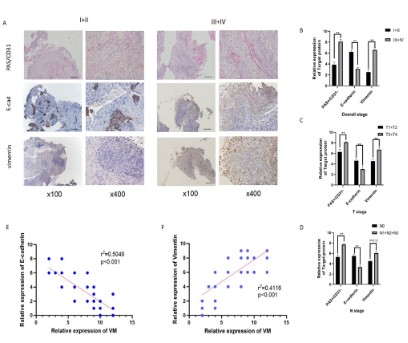
EMT was significantly positively correlated with VM in nasopharyngeal carcinoma clinical samples
We divided the tissue samples of 60 patients with nasopharyngeal carcinoma into stage I (4 cases), stage II (8 cases), stage
III (35 cases), and stage IV (13 cases). The detailed Immunohistochemical staining scores for each group are shown in Table 1. The
VM and E-cadherin and Vimentin sections stained are shown in
Figure 1A.
The association of VM and E-cadherin and Vimentin with clinical stage and TNM stage was analyzed according to immunohistochemical scores. VM indicators were higher in stage III-IV than in
stage I-II nasopharyngeal carcinoma. As the clinical stage of the
tumor increased, E calponin expression decreased, but vimentin
increased. The results suggest that the EMT process is promoted
(Figure 1B). Similar results were obtained in the analysis of outcomes in T-stage versus N-stage (Figure 1C,D).
We compare the relationship between EMT and VM. E-cadherin was significantly negatively correlated with VM in nasopharyngeal carcinoma tissues (r2=0.5049, p<0.001), while Vimentin was
positively correlated with VM (r2=0.4116, p<0.001) (Figure 1E,F).
This indicates that EMT is significantly positively correlated with
VM. The results suggest that EMT may be involved in the VM process.
Table 1: The relationship between VM expression, EMT and NPC
clinicopathological characteristics.
1. According to the 7th edition of the UICC/AJCC staging system.
| Characteristic |
Immunohistochemical staining score |
n |
| PAS+/CD31- |
E-cadherin |
vimentin |
| Overall stage1 |
|
|
|
|
| I+II |
3.917 ± 1.505 |
6.167 ± 2.167 |
2.500 ± 2.468 |
12 |
| III+IV |
8.167 ± 1.705 |
3.104 ± 2.146 |
6.625 ± 2.367 |
48 |
| P value |
<0.001 |
<0.001 |
<0.001 |
|
| Tumor stage1 |
| T1+T2 |
6.269 ± 2.662 |
4.654 ± 2.637 |
4.577 ± 2.982 |
26 |
| T3+T4 |
8.118 ± 1.805 |
3.000 ± 2.089 |
6.735 ± 2.478 |
34 |
| P value |
0.002 |
0.009 |
0.003 |
|
Node stage1 |
| N0 |
5.300 ± 3.591 |
5.500 ± 3.100 |
4.500 ± 3.837 |
10 |
| N1+N2+N3 |
7.720 ± 1.863 |
3.360 ± 2.183 |
6.060 ± 2.637 |
50 |
| P value |
0.003 |
0.011 |
0.120 |
|
|
|
|
|
0 |
EMT was positively correlated with VM formation ability in
vitro cell experiments
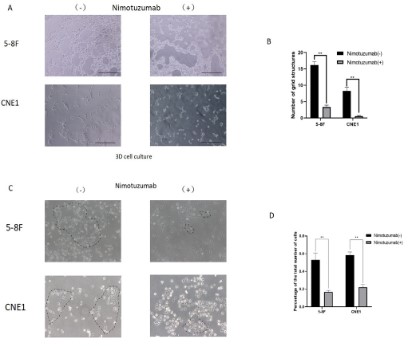
First, to investigate the effect of EGFR inhibitors on the ability
of nasopharyngeal carcinoma cells to form VM, we selected two
nasopharyngeal carcinoma cell lines: 5-8 F and CNE1. EGFR inhibitors significantly inhibit the tube-forming ability of tumor cells in
3D cell culture. The results suggest that EGFR inhibitors inhibit VM
formation (Figure 2A,B). Further observation of the ability of cells
to form characteristic tentacles (equivalent to the occurrence of
EMT markers). The results showed that after the addition of nimotuzumab, the proportion of "spindle cells" in 5-8 F and CNE1 cells
decreased significantly (5-8 F: 66%; CNE1: 60%), indicating that
the EMT process was inhibited (p<0.05) (Figure 2C,D).
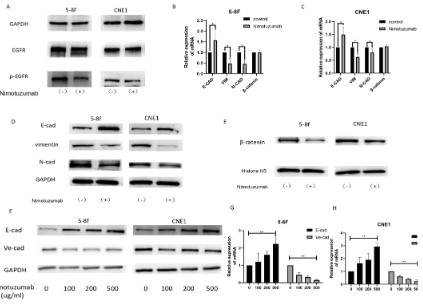
Then, we tested the effectiveness of nitumumab and found
that the expression of EGFR did not change significantly in the
experimental group (Nimotuzumab +) compared with the control
group (Nimotuzumab -), but the content of its phosphorylated
product p-EGFR was significantly decreased (Figure 3A).
Further, Further validation of the change in EMT index after
the addition of nitrozumab. The experimental results showed that
the gene expression and protein expression of E-cadherin in two
nasopharyngeal carcinoma cells increased (5-8 F: 56.6%; CNE1:
49.7%), while the gene and protein expression levels of Vimentin
and N-cadherin decreased (Vimentin 5-8 F: 52.4%, CNE1: 37.1%;
N-cadherin 5-8 F: 53.3%, CNE1: 20%), while the gene expression
level of β-catenin was almost unchanged (see Discussion section
for details) (Additional Figure 1). The above results suggest the
ability of EGFR inhibitors to inhibit epithelial mesenchymal transition in nasopharyngeal carcinoma.
To verify whether the inhibition of EMT and VM by Nimotuzumab is related to drug concentration, we designed a Nimotuzumab drug concentration gradient experiment. The experimental results showed that with the increase of nimotuzumab concentra-
tion, the protein content and gene expression level of E-cadherin
in the two cell lines gradually increased, while the protein content
and gene expression level of Ve-cadherin gradually decreased (Figure 3D-H).
In animal experiments, nimotuzumab can inhibit EMT process and VM formation
In our previous study, in order to investigate the relationship
between vasculogenic mimicry and Foxq1 and EGFR, we performed subcutaneous tumorigenesis experiments [16]. Brief description of the experiment: 5-8 F cells were injected subcutaneously
in nude mice. The tail vein of the experimental group was injected
with Nitrozumab and the tail vein of the control group was injected with saline after tumor formation. We re-sliced and stained
the tumor specimens utilizing previous animal experiments from
our experimental group.
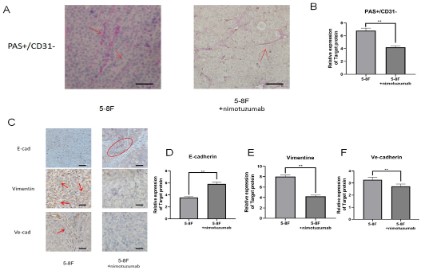
First, PAS/CD31 double staining was performed to detect the
formation of vasculogenic mimicry in tumor tissues. We found
that the tumor tissues of mice injected with nimotuzumab in the
tail vein were less prone to vasculogenic mimicry than those of
mice injected with normal saline (Figure 4A-B). Next, we examined EMT-related indicators (E-cadherin, Vimentin) and VM related indicators (VE-cadherin) in these animal tissues. We found
that e-cadherin expression was decreased and Vimentin and VE-cadherin expression was increased in nimotuzumab treated tumor tissues compared with saline treated mouse tumor tissues.
Nimotuzumab inhibited the EMT process and VM suppression
was also observed (Figure 4C-F).
Discussion
In this article, we verified the relationship between angiogenic
mimicry and EMT from tissue samples, cellular experiments and
animal experiments. The results suggest that EMT may act as a
bridge mediating the EGFR pathway and promote angiogenic mimicry in nasopharyngeal carcinoma.
Vasculogenic mimicry (VM) is a vascular-like structure which
can mimic the embryonic vascular network pattern to nourish the
tumour tissue [17]. As a unique perfusion way, VM is correlated
with tumour progression, invasion, metastasis and lower 5-year
survival rate. Notably, Epithelial-Mesenchymal Transition (EMT)
regulators and EMT-related transcription factors are highly up-
regulated in VM-forming tumour cells, which demonstrated that
EMT may play a crucial role in VM formation [18]. Therefore, the
up-regulation of EMT-associated adhesion molecules and other
factors can also make a contribution in VM-forming process [19].
Our study found a correlation between EMT and vasculogenic mimicry in nasopharyngeal carcinoma. When we inhibited the formation of vasculogenic mimicry vessels in nasopharyngeal carcinoma by using EGFR inhibitors, we detected that the EMT process
was inhibited. Our results found a strong correlation between
EMT and VM. It is highly likely that EMT is a bridge for VM formation. However, experiments are still needed to confirm their direct
relationship. We also added that the EGFR signaling pathway can
promote the formation of EMT and VM.
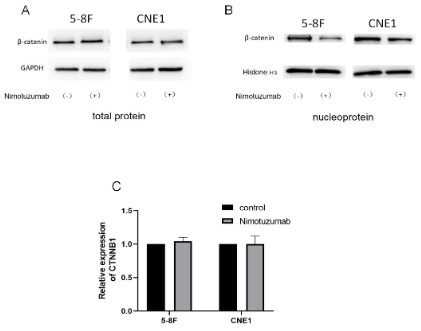
During the experiment, we discovered an interesting phenomenon. we began to extract the total protein of the cells to detect the expression of β-catenin. There was no difference in the
expression of β-catenin between the nimotuzumab-treated group
and the negative control group. Then we extracted the β-catenin
expression of the cell nucleus, and the beta-catenin expression
of the nimotuzumab treatment group decreased (Additional Figure 1). β-catenin is a multifunctional protein that helps cells
respond to signals and influences outside the cell by interacting
with the cytoskeleton. This protein acts as a transcription factor
in the nucleus and turns on genes that promote cell division. In
the absence of Wnt signaling, β-catenin is degraded by protein complexes including Axin, APC, Ser/Thr kinases GSK-3 and CK1,
protein phosphatase 2A (PP2A), and E3-ubiquitin ligase B-TrCP.
This complex specifies the B-TrCP recognition site on β-catenin by
phosphorylation of a conserved Ser/Thrrich sequence near the
amino terminal. Phosphorylation requires Axin to scaffold GSK-3
and CK1 and β-catenin. After phosphorylation and ubiquitination,
β-catenin is degraded by the proteasome. Binding of Wnt to its
receptor induces binding of Axin to phosphorylated lipoprotein
receptor-associated protein (LRP). The breakdown of the complex stabilizes β-catenin, which accumulates in the cytoplasm
and enters the nucleus, where it subsequently binds to TCF in the
nucleus, thereby upregulation of target genes [20]. In this study,
western blot analysis showed that the level of β-catenin in the
nucleus decreased (with histone H3 as internal reference) when
vasculogenic mimicry formation was inhibited. However, qRT-PCR
showed no change in the expression level of the CTNNB1 gene,
which regulates the expression of β-catenin. Therefore, we hypothesized that during EMT, the total amount of β-catenin did
not change significantly, but its intracellular distribution changed,
from cytoplasm to nucleus. The mechanism of β-catenin regulation of EMT and VM remains to be further studied.
Conclusions
EGFR-regulated EMT is a driver of vasculogenic mimicry in Nasopharyngeal Carcinoma.
Declarations
Ethics approval: All clinical studies were approved by the
Ethics Committee of Southern Medical University. All animal experiments were performed in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of
Southern Medical University.
Conflicts of interest: The authors declare no competing interests
Data availability: The data that support the findings of this
study are available from the corresponding author upon reasonable request.
Informed consent: Informed written consent was obtained
from each patient.
Funding: This study was supported by grants from the National
Natural Science Foundation of China (81702696), Natural Science
Foundation of Guangdong Province of China (2017A030310040
and 2020A1515010176), and Supported by Beijing xisike Clinical
Oncology Research Foundation.
References
- Hernández De La Cruz ON, López-González JS, García-Vázquez R,
Salinas-Vera YM, Muñiz-Lino MA, et al. Regulation Networks Driving Vasculogenic Mimicry in Solid Tumors. Frontiers in Oncology.
2020; 9.
- Krishna Priya S, Nagare RP, Sneha VS, Sidhanth C, Bindhya S, et al.
Tumour angiogenesis-Origin of blood vessels. International Journal of Cancer. 2016; 139: 729-735.
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, et al. Vascular channel formation by human melanoma cells in vivo and in
vitro: vasculogenic mimicry. Am J Pathol. 1999; 155: 739-752.
- Seftor REB, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, et al.
Tumor Cell Vasculogenic Mimicry. The American Journal of Pathology. 2012; 181: 1115-1125.
- Andreucci E, Peppicelli S, Ruzzolini J, Bianchini F, Calorini L et al.
Physicochemical aspects of the tumour microenvironment as
drivers of vasculogenic mimicry. Cancer and Metastasis Reviews.
2022.
- Lezcano C, Kleffel S, Lee N, Larson AR, Zhan Q, et al. Merkel cell
carcinoma expresses vasculogenic mimicry: demonstration in patients and experimental manipulation in xenografts. Lab Invest.
2014; 9: 1092-102
- Bhuniya A, Sarkar A, Guha A, Choudhury PR, Bera S, et al. Tumor
activated platelets induce vascular mimicry in mesenchymal stem
cells and aid metastasis. Cytokine. 2022; 158: 155998.
- Song X, An Y, Chen D, Zhang W, Wu X, et al. Microbial metabolite
deoxycholic acid promotes vasculogenic mimicry formation in intestinal carcinogenesis. Cancer Science. 2022; 113: 459-477.
- Carroll CP, Bolland H, Vancauwenberghe E, Collier P, Ritchie AR et
al. Targeting hypoxia regulated sodium driven bicarbonate transporters reduces triple negative breast cancer metastasis. Neoplasia. 2022; 25: 41-52.
- Riemann A, Rauschner M, Gießelmann M, Reime S, Haupt V, et
al. Extracellular Acidosis Modulates the Expression of Epithelial-Mesenchymal Transition (EMT) Markers and Adhesion of Epithelial
and Tumor Cells. Neoplasia, 2019; 21: 450-458.
- Riemann A, Rauschner M, Gießelmann M, Reime S, Thews O, et
al. The Acidic Tumor Microenvironment Affects Epithelial-Mesenchymal Transition Markers as Well as Adhesion of NCI-H358 Lung
Cancer Cells. Adv Exp Med Biol. 2021; 1269: 179-183.
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nature Reviews Molecular Cell Biology.
2014; 15: 178-196
- Zhao J, Wu J, Qin Y, Zhang W, Huang G, et al. LncRNA PVT1 induces
aggressive vasculogenic mimicry formation through activating the
STAT3/Slug axis and epithelial-to-mesenchymal transition in gastric cancer. Cellular Oncology. 2020; 43: 863-876.
- Li W, Zhou Y. LRIG1 acts as a critical regulator of melanoma cell
invasion, migration, and vasculogenic mimicry upon hypoxia by regulating EGFR/ERK-triggered epithelial–mesenchymal transition.
Bioscience Reports. 2019; 39.
- Chen YP. Nasopharyngeal carcinoma. Lancet. 2019; 394: 64-80.
- Luo Y, Wang J, Wang F, Liu X, Lu J, et al. Foxq1 promotes metastasis
of nasopharyngeal carcinoma by inducing vasculogenic mimicry
via the EGFR signaling pathway. Cell Death & Disease. 2021; 12.
- Luo Q, Wang J, Zhao W, Peng Z, Liu X, et al. Vasculogenic mimicry
in carcinogenesis and clinical applications. Journal of Hematology
& Oncology. 2020; 13.
- Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis.
Journal of Hematology & Oncology. 2022; 15.
- Liu Q, Qiao L, Liang N, Xie J, Zhang J, et al. The relationship between
vasculogenic mimicry and epithelial-mesenchymal transitions.
Journal of Cellular and Molecular Medicine. 2016; 20: 1761-1769.
- Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and
Emerging Therapeutic Modalities. Cell. 2017; 169: 985-999.