Introduction
Anaesthesia for gynaecological surgeries could be general, epidural, spinal or combined spinal-epidural anaesthesia [1]. Open abdominal myomectomy and hysterectomy are major types of gynaecological procedures and their anaesthesia poses some challenges, especially in developing countries due to socioeconomic factors like poverty, illiteracy, unavailability of anaesthetic drugs and insufficient number of trained Physician Anaesthetists [2,3]. Takai et al [2] found that a high number of the procedures are done under general anaesthesia, but they also noted the use of regional anaesthesia. In another study conducted by Nnaji et al [3], it was observed that combined spinal-epidural (CSE) anaesthesia offers some benefits in terms of better intraoperative and postoperative analgesia in abdominal Myomectomy, but the utilisation rate was very low (1.5%).
General anaesthesia offers better relaxation for gynaecological procedures; however, it could be associated with airway mishaps [4]. While spinal anaesthesia offers good analgesia in an awake patient, most time it does not provide anaesthesia long enough to last the duration of the surgery. Epidural anaesthesia, which is often under utilised in our environment, can offer both intraoperative and postoperative analgesia, and it has the potential to reduce or eliminate the perioperative physiologic stress response to surgery and thereby decrease surgical complications and improve outcomes [5]. Epidural anaesthesia is usually administered for surgeries in the lower abdomen, perineum and lower extremities. Although its onset of action is slow, and sometimes associated with patchy sensory blocks, when properly performed, it can offer good anaesthesia and outlast the duration of prolonged major gynaecological surgeries like abdominal myomectomy and hysterectomy [6,7].
Epidural anaesthesia can be administered as single shot, intermittently, continuously or as patient controlled injection. Some authors have evaluated the addition of adjuvants like dexmedetomidine, clonidine, morphine, fentanyl and neostigmine to local anaesthetic like bupivacaine for epidural anaesthesia in an attempt to potentiate and prolong the analgesic effect [8,9]. However, consideration should also be made to evaluate the haemodynamic effect of combination of local anaesthetics with adjuvants and the techniques of epidural anaesthesia.
There is a perception that continuous epidural infusion of local anaesthetic produces an unchanging block to maintain analgesia and minimise cardiovascular disturbance [10], but this has not been exclusively evaluated. Thus, we conducted a prospective study with the primary aim of determining the differences in the intraoperative haemodynamic changes using continuous or intermittent epidural injection of local anaesthetic agents with opioids. The secondary outcome measures were to determine the differences in the maximum level of sensory block, total volume of epidural drug injection, and side effects that can occur between the two methods of epidural administration.
Methodology
Dorsal hand-foot syndrome (dorsal HFS) is an adverse reaction
induced with taxanes, mainly characterizes as symmetrical,
pleomorphic purplish red plaques, pigmentation, desquamation,
pain, itching and swelling in the skin of the back of the hand and
foot, which can occasionally involve the palmoplantar part [1-4]. In
reviewing the literatures of chemotherapy related dermal toxicity,
dorsal HFS is generally caused by paclitaxel and docetaxel [1,2,5,
6]. And the incidence of docetaxel-induced HFS is approximately
10%, more common than paclitaxel [7]. However, there are no
covers about nab-paclitaxel induced with dorsal HFS. Furthermore,
this untoward dermal toxicity on the dorsal skin of hands and feet
may be classified as HFS in some case reports. At present, there
is no systematic descriptions of the pathogenesis, diagnosis and
treatment of dorsal HFS. In this review, we will present a case of dorsal hand-foot syndrome that induced by nanoparticle albuminbound paclitaxel, and will discuss from the aspects of differential
diagnosis, possible pathogenesis and managements.
Case presentation
A 75-year-old female with advanced gastric cancer and
peritoneal metastasis was admitted to our hospital. In terms
of treatment scheme selection, according to multi-disciplinary
team’s advice, she was recommended to use chemotherapeutic
drugs to treat illness. And since July 14,2020, this patient has been
treated with nab-paclitaxel combined tegafur (Table 1). And prior
to each period, she was premedicated with 5 mg dexamethasone,
5 mg tropisetron, 50 mg diphenhydramine, and 150 mg fosapitan.
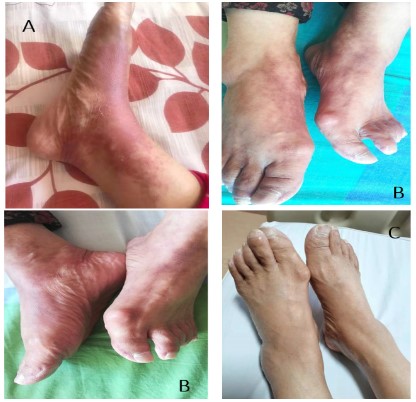
On cycle 6 day 14, the patient complained of pain with erythema
or violaceous papules, pruritus, swelling and paresthesia on her dorsum of feet, that prevented her from pursuing activities of
daily life (Figure 2A). To alleviate these unwell symptoms, she
received palliative therapies in another hospital and subsequently
returned to our hospital one month later than originally scheduled.
After admission, she underwent physical examination and results
revealed tender erythema, purplish red papules and plaques,
mildly desquamation and edema on her dorsal feet skin (Figure
2B). She denied the history of taking other drugs and contacting
allergens during chemotherapy. Combined with the patient’s
clinical symptoms and treatment process, in order to determine
whether this series of symptoms of the patient are adverse
reactions of chemotherapy drugs or diseases of blood system, the
patient was further tested by routine blood test, liver function test
and coagulation test, and no abnormality was found in the test
results (Table 2). Therefore, subcutaneous hemorrhage caused by
diseases of blood system was excluded, and skin toxicity caused
by chemotherapy drugs was considered. And her symptoms were
more likely to be considered as dorsal hand-foot syndrome, which
caused by nab-paclitaxel.
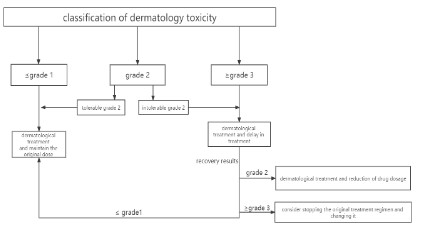
According to the grade of toxic and side effects of
chemotherapeutic drugs, her symptoms were considered as grade
3 or 4 (Table 3) Adverse Event (AE) [8]. Due to the efficacy of the
current chemotherapy regimen was evaluated as stable disease
(shrinkage), so according to AE treatment strategies (Figure 2),
we withdrew albumin-paclitaxel and selected tegafur as her
maintenance treatment [7,9]. She was also advised to undergo
palliative measures by taking low dose corticosteroids, using
hirudoid cream, wearing soft clothes, and reducing skin friction
on feet. We also followed up with this patient. During the followup period, these uncomfortable reactions on the patient’s dorsum
of feet gradually improved, and after the sixth courses of teggio
maintenance treatment, her feet skin returned to be normal
(Figure 2C). The patient’s follow-up results further confirmed that
albumin-paclitaxel was the offending agent.
Table 1: The patient’s treatment strategy and treatment course.
| Cycle |
Day |
Treatment |
| Cycle 1-2 |
D1 |
albumin-bound paclitaxel 260 mg/m² peritoneal perfusion |
| D8 |
albumin-bound paclitaxel 260 mg/m² intravenous |
| D1-D14 |
Tegafur 80mg/m² ora |
| Cycle 1-2 |
D1 |
albumin-bound paclitaxel 260 mg/m² intravenous |
| D8 |
albumin-bound paclitaxel 260 mg/m² intravenous |
| D1-D14 |
Tegafur 80mg/m² oral |
Table 2: The patient’s laboratory test results.
| December 12,2020 |
| Blood routine |
RBC: 3.78 x 1012/L |
HGB: 114 g/L |
PLT: 192 x 109/L |
PLT: 192 x 109/L |
|
|
| Coagulation routine |
TT: 17.0s |
APTT: 26.4s |
PT: 10.4s |
INR: 0.88 |
PT |
FB |
|
|
|
|
|
A: 130% |
G: 4.17 g/L |
| Liver function |
TBIL: 9.1 umol/L |
DBIL: 1.7 umol/L |
IBIL: 7.4 umol/L |
Globulin: 24.7 g/L |
|
|
Table 3: Classification criteria of HFS according to NCI and WHO.
| Grade |
NCI |
WHO |
| 1 |
Minimal skin changes or dermatitis (rash, edema, hyperkeratosis) without pain |
Dysesthesia/paresthesia, tingling in hands and feet |
| 2 |
Skin changes (peeling, blisters, bleeding, cracks, edema, hyperkeratosis) with
pain, limiting instrumental ADL |
Discomfort in holding objects or in walking, edema or/and erythema
without pain |
| 3 |
Severe skin changes (peeling, blisters, bleeding, cracks, edema, hyperkeratosis)
with pain; limiting self-care ADL |
Painful erythema and edema in palms and soles, and around fingernails
and toenails |
| 4 |
|
Desquamation, ulceration, blistering, severe pain |
NCI: the American National Cancer Institute; WHO: the World Health Organization; ADL: activities of daily life.
Discussion
Among the side effects related to chemotherapeutic drugs,
dermatological toxicity is the most common adverse reaction.
Common cutaneous adverse reactions include eruption, HandFoot Syndrome (HFS), dorsal hand-foot syndrome, Periarticular
Thenar Erythema With Onycholysis (PATEO), and Hand Foot Syndrome Reaction (HFSR). Hand-Foot Syndrome (HFS), one of the
most universal adverse effects commonly characters as palmoplantar numbness, tingling, burning pain, erythema, pigmentation, with or without edema on palms and soles, can be induced
with a variety of chemotherapeutic agents, such as capecitabine,
doxorubicin, 5fluorouracil, taxanes and so on [10-12]. Compared
with HFS, the incidence of dorsal hand-foot syndrome is much
lower.
Albumin-bound paclitaxel (nab-paclitaxel), a novel, solventfree taxane drug, which has demonstrated advantages in delivering a higher dose of paclitaxel to foci and reducing the incidence
of severe adverse reactions. The common toxicities of nab-paclitaxel include anaphylactic reactions, myelosuppression, mucositis, fatigue and neuropathy [13].
Compared to paclitaxel and docetaxel, albumin-paclitaxel
caused very few dermal toxicity reactions. Here, we report a case
of dorsal HFS induced by nab-paclitaxel rather than tegeo-guided
HFS. After withdrawing albumin paclitaxel, the patient’s discomfort gradually eliminated and the rash did not recur. The follow-up
result of this patient also authenticated that the skin toxicity was
caused by albumin paclitaxel. This further reminds us that timely
and accurately identify dorsal HFS is crucial for the choice of subsequent treatment. However, there is no systematic description
about dorsal hand-foot syndrome from clinical symptoms, possible pathogenesis and effective managements.
First of all, in terms of diagnosis, dorsal hand and foot syndrome should be distinguished from other cutaneous toxic reactions induced with chemotherapeutic drugs, such as HFS, PATEO,
HFSR and so on. In clinical symptoms characterizes: hand-foot
syndrome is initially manifested as palmoplantar numbness, tingling, burning pain, and then erythema, with or without edema,
desquamation. HFS is mainly founded on palms and soles that
may be related to the large concentration of microcapillaries and
sweat glands in these areas. PATEO showed purplish red plaques
at the protrusions of large and small fish and on dorsum of hands,
accompanied with nail changes that frequently progress to onycholysis. However, dorsal HFS mainly characters as symmetrical, pleomorphic purple patches, pigmentation, desquamation, with
or without pain, itching and swelling appeared in the affected
parts. It focuses on the dorsum of hands and rare on the dorsum
of feet or around the ankle that may be related to sun-exposed [7,
14-17]. In addition to distinguishing from other dermatology toxicity caused by chemotherapeutic drugs, dorsal HFS should also
be distinguished from subcutaneous bleeding caused by blood
system diseases, such as thrombocytopenic purpura, leukemia
and so on. They can be identified by perfecting laboratory related tests. As for histopathological characteristics, relevant studies
have shown that HFS and dorsal HFS are similar. HFS’s histopathological feature is hyperkeratosis of the overlying epidermis,
spongiotic changes, focal vacuolization with necrotic and dyskeratotic keratinocytes in the basal cell layer. The histopathological
characteristics of dorsal HFS is Keratinocyte apoptosis, dyskeratosis, atypical mitotic figures, abnormal maturation of keratinocytes [1,18,19]. In terms of occurrence mechanism, different chemotherapy drugs cause HFS by different mechanisms. The exact
mechanisms governing HFS and dorsal HFS are unclear. Possible
pathogenesis of HFS are as follows: C0X-2-mediated inflammatory
response; chemotherapeutic drugs accumulated in small sweat
ducts; enzymes related to catabolism of chemotherapeutic drugs,
such as thymidine phosphorylase and Dihydropyridine Dehydrogenase (DPD) that are related to disassemble 5-FU; microcapillary
damage leading to drugs extravasation and consequent dermal
toxicity [14,15,20]. However, the mechanisms of dorsal HFS have
not been systematically studied.
According to the types of skin toxic reactions caused by taxol,
the possible pathogenesis are inflammatory reactions, solvent
response and the direct cytotoxic effect. The optimal therapeutic
for HFS and dorsal HFS has not yet been determined. Currently,
all dermatology toxicities’ managements mainly involve three aspects: patient’s self-monitoring, drug treatment and chemotherapeutic dose management [21,22]. During the entire treatment,
patients are advised to wear suit clothes, avoid strenuous exercise, refrain from injury and fraction of hands and feet. Except essential ways, patients with dorsal HFS are recommended to wear
ice gloves, caps and avoid sun exposure [22]. In terms of symptomatic treatment measures, patients may relieve discomfort by locally using urea cream, taking medicines such as COX-2 inhibitors,
pyridoxine, topical corticosteroids, vitamin E and herbal remedies
[23-26]. Chemotherapeutic dose management means that when
symptoms remain severe after symptomatic treatment, dose reduction or chemotherapy discontinuation may be considered
[7,9]. Although the managements of other cutaneous adverse effects and dorsal HFS are similar, enhanced awareness of how to
identify and treat dorsal HFS is crucial for prompt treatment and
subsequent options.
Conclusion
In conclusion, this is the first case presentation of dorsal handfoot syndrome developed by nab-paclitaxel. Currently, the mechanism and specific therapeutic measures for dorsal hand-foot
syndrome have not been precisely defined, more further studies
are needed to solve these problems.
How to promptly identify dorsal hand foot syndrome is pretty
essential to improve the quality of life and provide duly treat strategies for patients receiving chemotherapy.
Declarations
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author is responsible for all
aspects of the work to ensure that issues related to the accuracy
or completeness of any part of the work are properly investigated
and resolved. This case report has passed the ethical approval of
the First Hospital of Jilin University, and there is no conflict of interest when writing this article.
References
- Brandon Z, Kristin L, Jeave R, Jodi S. A Paclitaxel-Induced Variant
of Hand-Foot Syndrome Affecting Dorsal Surfaces. Journal of cutaneous pathology. 2021; 48.
- Patel J, Ringley JT, Moore DC. Case series of docetaxel-induced
dorsal hand–foot syndrome. Therapeutic Advances in Drug Safety.
2018; 9.
- Plummer RS, Shea CR. Dermatopathologic effects of taxane therapy. Journal of the American Academy of Dermatology. 2010; 65.
- C. ZG. Acute cutaneous reactions to docetaxel, a new chemotherapeutic agent. Archives of Dermatology. 1995; 131.
- Mirna F, Nagi E-S, Ali S. Hand-foot syndrome with docetaxel: A fivecase series. Annals of Saudi Medicine. 2008; 28.
- S KP, P KP, A PA, Nahush T, Vijay B, Honey P. Rare occurrence of
hand-foot syndrome due to paclitaxel: A rare case report. Indian
journal of pharmacology. 2018; 50.
- Vincent S, R LN, Henri R, R BV, Laurence G, Marion D, et al. Dermatological adverse events with taxane chemotherapy. European
journal of dermatology: EJD. 2016; 26.
- Nagore E, Insa A, Sanmartín O. Antineoplastic therapy-induced
palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. Incidence, recognition and management. Am J Clin Dermatol. 2000; 1:
225-234.
- Nikolaou V, Syrigos K, Saif MW. Incidence and implications of chemotherapy related hand-foot syndrome. Expert Opin Drug Saf.
2016; 15: 1625-1633.
- Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, et al.
Capecitabine, bevacizumab, and mitomycin in first-line treatment
of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin
Oncol. 2010; 28: 3191-3198.
- Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl
J Med. 2017; 376: 2147-2159.
- Tagawa N, Sugiyama E, Tajima M, Sasaki Y, Nakamura S, et al. Comparison of adverse events following injection of original or generic
docetaxel for the treatment of breast cancer. Cancer Chemother
Pharmacol. 2017; 80: 841-849.
- Fei H, Jiaxuan L, Xin S, Zijing W, Qiao L, et al. Adverse Event Profile
for Nanoparticle Albumin-Bound Paclitaxel Compared With Solvent-Based Taxanes in Solid-Organ Tumors: A Systematic Review
and Meta-Analysis of Randomized Clinical Trials. The Annals of
pharmacotherapy. 2021.
- Martschick A, Sehouli J, Patzelt A, Richter H, Jacobi U, et al. The
pathogenetic mechanism of anthracycline-induced palmar-plantar
erythrodysesthesia. Anticancer Res. 2009; 29: 2307-2313.
- Lou Y, Wang Q, Zheng J, Hu H, Liu L, Hong D, et al. Possible Pathways of Capecitabine-Induced Hand-Foot Syndrome. Chem Res
Toxicol. 2016; 29: 1591601.
- Yokomichi N, Nagasawa T, Coler-Reilly A, Suzuki H, Kubota Y, et al.
Pathogenesis of Hand-Foot Syndrome induced by PEG-modified
liposomal Doxorubicin. Hum Cell. 2013; 26: 8-18.
- Rzepecki AK, Franco L, McLellan BN. PATEO syndrome: periarticular thenar erythema with onycholysis. Acta Oncol. 2018; 57: 991-
992.
- Antonio C, Tecla T, Gladys H-T, George K. Paclitaxel-induced neutrophilic adverse reaction and acral erythema. Acta dermato-venereologica. 2011; 91.
- Gordon KB, Tajuddin A, Guitart J, Kuzel TM, Eramo LR, et al. Handfoot syndrome associated with liposome-encapsulated doxorubicin therapy. Cancer. 1995; 75: 2169-2173.
- Van Kuilenburg AB, Meinsma R, Zoetekouw L, Van Gennip AH. Increased risk of grade IV neutropenia after administration of 5-fluorouracil due to a dihydropyrimidine dehydrogenase deficiency:
high prevalence of the IVS14+1g>a mutation. Int J Cancer. 2002;
101: 253-258.
- Johannes J M K. Management of cytotoxic chemotherapy-induced
hand-foot syndrome. Oncology reviews. 2020; 14: 442.
- Jiexiu C. How to conduct integrated pharmaceutical care for patients with handfoot syndrome associated with chemotherapeutic
agents and targeted drugs. Journal of oncology pharmacy practice
: official publication of the International Society of Oncology Pharmacy Practitioners. 2021; 27: 919-929.
- Chen LT, Han TP, Wai TK, Wei HT. Effect of Urea Cream on HandFoot Syndrome in Patients Receiving Chemotherapy: A Meta-analysis. Cancer Nursing. 2021.
- Rong-Xin Z, Xiao-Jun W, Shi-Xun L, Zhi-Zhong P, De-Sen W, Gong C.
The effect of COX-2 inhibitor on capecitabine-induced hand-foot
syndrome in patients with stage II/III colorectal cancer: a phase
II randomized prospective study. Journal of cancer research and
clinical oncology. 2011; 137.
- . Lian S, Zhang X, Zhang Y, Zhao Q. Pyridoxine for prevention of
hand-foot syndrome caused by chemotherapy agents: a meta-analysis. Clin Exp Dermatol. 2021; 46: 629-635.
- Bozkurt Duman B, Kara B, Oguz Kara I, Demiryurek H, Aksungur E.
Hand-foot syndrome due to sorafenib in hepatocellular carcinoma
treated with vitamin E without dose modification; a preliminary
clinical study. J buon. 2011; 16: 759-764.