Background
Crataegi fructus is the dried and mature fruit of crataegus
pinnatifid a. bge. var. major n. e. br. of Rosaceae or Crataegus
pinnatifida Bge, with the effects of «promoting digestion, invigorating stomach, moving qi, removing blood stasis, turbidity and
lipid-lowering» etc [1]. Modern studies have shown that it mainly
contains flavonoids and their glycosides, polyphenols, triterpenoids and organic acid compounds and other active components.
Previous studies have shown that Crataegi fructus has a wide
range of pharmacological effects, the Crataegi fructus extract has
a significant hypoglycemic and lipid lowering effect [2-4]. SHIH et
al. [5] gavaged a certain amount of aqueous extract of Crataegi
fructus into mice and found that it could reduce glucose production and triglyceride synthesis and improve insulin resistance by
inducing phosphorylation of AMPK. AIERKEN et al. [3] showed
that Crataegi fructus extract not only reduced the blood glucose
level of Type 2 diabetes mellitus (T2DM) model mice, but also increased the release of pancreatic plasma insulin level.
In addition, modern pharmacological studies have shown that
Crataegi fructus extract can effectively reduce blood lipid levels
in rats, inhibit oxidative stress, and improve body fat deposition
[6,7]. However, due to the unclear specific components and targets, there has been no in-depth study on lowering blood glucose
and regulating lipid, which has affected the exertion of its advantages to a certain extent. Therefore, in this study, the network
pharmacology was used to systematically explore the health-care
efficacy of Crataegi fructus in lowering blood glucose and regulating lipid, in order to further identify the potential action mechanism and pharmacodynamic material basis, and provide further
reference for the application of Crataegi fructus and the development of health-care functional foods.
Network pharmacology is an emerging discipline that connects
drugs and diseases from a systematic and holistic perspective
by constructing the network relationship of «drug-componentdisease-target-pathway». It is highly consistent with the overall
concept and dialectical treatment thought of Traditional Chines e
medicine (TCM), and is a new method for the development and
modernization of traditional Chinese medicine [8]. In view of this,
this study made an in-depth and systematic study on the potential molecular mechanism of hypoglycemic and lipid-lowering of
Crataegi fructus from the perspective of network pharmacology,
in order to provide a reference for further development and applicati on of Crataegi fructus.
Materials and methods
Acquisition of Crataegi fructus components and prediction of
their targets
The Crataegi fructus components were obtained from TCMID
(http://www.megabionet.org/tcmid/) [9] and BATMAN (http://
bionet.ncpsb.org/batman-tcm/) databases [10], and the corresponding targets of the compounds were obtained from (TCMSP)
(http://tcmspw.com/tcmsp.php) [11] and Batman databases. At
the same time, the protein database Uniprot (http://www.uniprot.org/uploadlists/) [12] was used to convert them into unified
gene names.
Identification of glucose-lowering and lipid-regulating targets
of Crataegi fructus
Gene Cards (https://www.genecards.org/) database is a platform that can provide all known human genes in genome, protein group, transcription, heredity and function [13]. Using «Diabetes» and «Hyperlipidemia» as keywords, the target information
related to hyperlipidemia and diabetes was collected. The target
points of hyperlipidemia, diabetes and Crataegi fructus, which
were searched in Genecards database, were mapped in Venny
2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/), and their common targets were screened out as potential targets for lowering
blood sugar and regulating lipid of Crataegi fructus.
Construction of the «Ingredient-Target-Disease» network and
screening of key targets
To identify the interaction between the hypoglycemic targets
and lipid-lowe ring targets of Crataegi fructus, the screened targets were introduced into the S TRING network platform (https://
string-db.org/) to construct a protein-protein interaction (PPI, the
process of combining two or more proteins to determine their
biochemical functions) network. The protein type was set as
«Homo sapiens», the scoring condition (confidence level) was set
as >0.9, and the PPI network format file of the target was exported. The active components, their corresponding Crataegi fructus
hypoglycemic and lipid-lowering targets and PPI were in putted
into Cytoscape 3.2.1 to construct a «component-target-disease»
network for hypoglycemic and lipid regulating of Crataegi fructus,
and the topology analysis of the network was performed using
the «Network Analyzer» plug-in. (Degree, DG) and (Between ness
Centrality, BC) and (Closeness Centrality, CC) are important topological parameters for evaluating the proportion of a node in a
network. DG can reflect the number of links between a node and
other nodes in the network. BC reflects the ratio of the number of
paths passing through the node to the total number of shortest
paths in all the shortest paths in the network. CC is used to measure the importance of nodes. Therefore, the larger the topology
parameter of the node is, the more critical the target is in the
network [14].
KEGG signal pathway and GO biological process enrichment
analyses
The core targets of Crataegi fructus identified in «1.2» and
«1.3» were analyzed for GO biological process and enrichment of
KEGG signaling pathway u sing DAVID database. The target genes
with P<0.05 were screened to obtain the main signaling pathways
and biological processes involved in the efficacy of Fructus Crataegi.
Molecular docking assisted validation
The protein structure file was downloaded from the Protein
Data Bank (PDB) website (https://www.rcsb.org/), and the target
protein and component were pre-treated using Auto dock 1.5.6
and Discovery Studio 2.5 software. Then Auto dock vina was used
to perform molecular docking on the targets and components.
The binding energy (affinity, kcal/mol) ≦5 was considered to be
the better binding, and the binding energy ≦7 was considered to
be the better binding [15,16].
Results and analysis
Main components and corresponding targets of Crataegi
fructus
The 82 components of Crataegi fructus were obtained from
the two databases, of which 58 components had action targets.
After correction and removal of duplicates by Uniprot, a total of
575 targets for Crataegi fructus were obtained from the databases of TCMSP and BATMAN.
Component–target network of Crataegi fructus for hypoglycemic and lipid-lowering
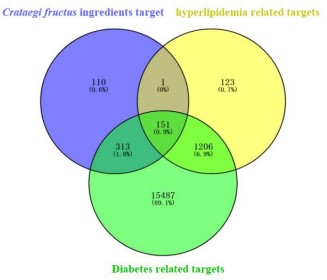
A total of 1481 and 17157 targets related to hyperlipidemia
and diabetes mellitus were obtained from Gene cards database.
These targets were mapped to 152 and 464 targets in Venny 2.1.0,
respectively. These targets are potential targets for Crataegi fructus hypoglycemic and lipid-lowering.
Screening of key targets of Crataegi fructus for hypoglycemic
and lipid regulation
In order to better explore the action mechanism of Crataegi
fructus in lowering blood glucose and regulating lipid, the related
targets of Crataegi fructus in lowering blood glucose and regulating lipid were input into the STRING database to obtain a target PPI network file, and the active components and target PPI
were imported into Cytoscape software to remove the isolated
components that did not intersect with the targets, and the target network PPI of Crataegi fructus in lowering blood glucose and
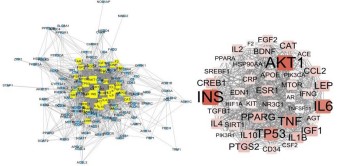
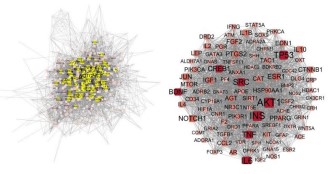
regulating lipid was drawn (Figures 2,3). The topological analysis
results of Crataegi fructus hypoglycemic network showed that
the median values of DG, CC and BC were 20, 0.415 and 0.001,
respectively, and a total of 94 targets met the screening requirements. The results of topology analysis of Crataegi fructus lipid regulatory network showed that the median values of DG, CC and
BC were 31, 0.488 and 0.002, respectively, and a total of 42 targets met the screening requirements. In addition, 94 key targets
of Crataegi fructus for lowering blood glucose included 42 potential targets of Crataegi fructus for regulating blood lipid, and the
first five targets of the network were all INS, AKT1, IL6, TP53, and
TNF, indicating that blood glucose was closely related to the occurrence and development of blood lipid and they affected each
other, suggesting that these targets were the key to lowering
blood glucose and regulating blood lipid of Fructus Crataegi.
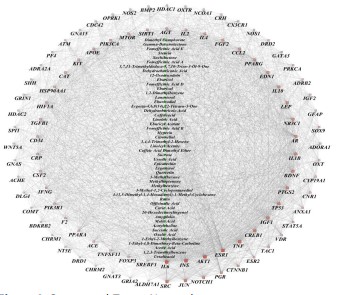
The composition - target network of Crataegi fructus is shown
in figure 4, in which the triangle represents the Crataegi fructus
components and the quadrilateral represents the Crataegi fructus
hypoglycemic and lipid-lowering targets. The inner and outer rings
are both potential targets of Crataegi fructus hypoglycemic and lipid-lowering, and the inner rings are potential targets of Crataegi
fructus lipid-lowering. Network topology analysis showed that
the average connectivity value, betweenness and near-centrality
of the compounds were 5,0.0000459 and 0.43, respectively, indicating that Crataegi fructus compound acted on multiple targets.
Through network topology parameter screening, there were four
important active ingredients, including kahenol (DG= 55), quercetin (DG=21), epicatechin (DG=15) and ursolic acid (DG=14). It
was speculated that the above active ingredients were the key
components of of Crataegi fructus for lowering blood sugar and
regulating lipids.
GO biological process analysis
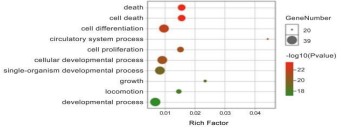
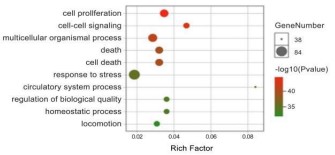
The GO biological process enrichment analysis was performed
on the key targets of Crataegi fructus hypoglycemic and lipid regulation, and the top 10 biological processes with P<0.05 were
screened out. The visualization results are shown in figures 5,6.
These targets are mainly involved in biological processes such as
the intervention of immune system processes, cell proliferation
and apoptosis and invasion, toxic metabolism and cytokine activity and regulation of their synthesis processes, which are the
biological processes in which the key targets of Crataegi fructus
hypoglycemic and lipid regulation are involved. Crataegi fructus
may play a role in lowering blood sugar and regulating lipids by
intervening in the above biological processes.
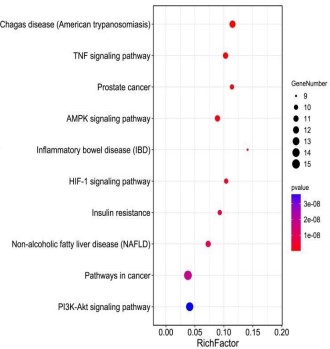
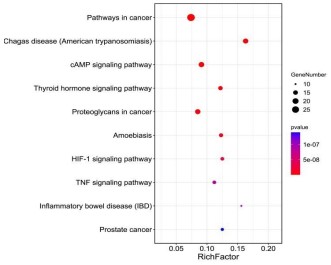
KEGG signal pathway enrichment analysis
Biological pathways perform specific biological functions
through the interactions between the different target proteins
they constitute, and are the physiological basis for understanding
the clinical manifestations of diseases. Therefore, drug intervention in disease is not only related to target proteins, but also affected by the biological pathway in which the target proteins are located, and the disordered body can be restored to balance through
the interaction result, especially for traditional Chinese medicine
and compound prescriptions with multi-component, multi-target
and multi-pathway characteristics. Therefore, in this study, KEGG
signal pathway enrichment analysis was conducted on key targets
of Crataegi fructus hypoglycemic and lipid-lowering respectively,
and the signaling pathways with P<0.05 were screened out, and
the first ten pathways were visualized, as shown in figures 7,8. As
shown in the bubble chart, Crataegi fructus is mainly involved in
PI3K-AKT, HIF-1, AMPK, TNF, and INS signaling pathways, among
which PI3K-AKT signaling pathway enriched in diabetes is the
most significant, while TNF and HIF-1 signaling pathways enriched
in hyperlipidemia are the most significant. These results indicated that Fructus Crataegi mainly focused on interfering with PI3KAKT signaling pathway to achieve the hypoglycemic effect, and
its lipid-lowering mechanism mainly focused on inter fering with
TNF and HIF-1 signaling pathways. Therefore, the above pathways
were most likely to be the key pathways for Crataegi fructus to
achieve lipid -lowering and hypoglycemic health effects.
Molecular docking assisted validation
In the composition target network, the top five components
with DG value included kaempferol (flavonoids), quercetin (flavonoids), ursolic acid (triterpenoids) and epicatechin (polyphenols).
Therefore, the above five targets were selected in the present
study to further explore the material basis of Crataegi fructus for
lowering blood glucose and regulating lipid. The docking results
showed that each component had different degrees of binding to
each target (Table 1), and there were 4, 3, 2, 1 and 4 compounds
with binding energies less than -7.0 kcal/mol with INSR, AKT1, IL6,
TP53 and TNF-α, respectively. The binding energies of quercetin
to five targets were all less than -7.0 kcal/mol. The best targets
combining with the components included INSR, AKT1, and TNF-α,
indicating that these components all contributed to different degrees in the process of glucose and lipid reduction and regulation
of Crataegi fructus. The above targets may play a key role in the
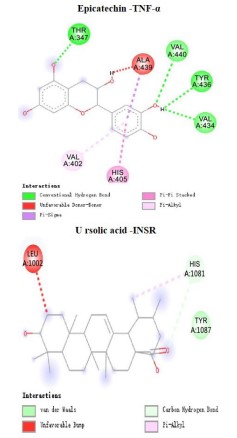
health care of hypoglycemic and lipid regulation in Crataegi fructus. Figure 9 shows the optimal interaction of each component
with the target.
Table 1: Binding energies of kenerl compounds and targets.
| Compound |
Target |
Binding energy/kcal/mol |
compound |
Target |
Binding energy/kcal/mol |
| Kaempferol |
INSR |
-8 |
Quercetin |
INSR |
-7.9 |
| AKT1 |
-8.2 |
AKT1 |
-8.7 |
| IL6 |
-6.6 |
IL6 |
-7.4 |
|
TP53 |
-6.7 |
|
TP53 |
-7.8 |
| TNF-α |
-8.3 |
TNF-α |
-9 |
| INSR |
-7.3 |
INSR |
-7 |
| AKT1 |
-8.5 |
AKT1 |
-4 |
| Epicatechins IL6 |
-6.5 |
Ursolic acid IL6 |
-7 |
| TP53 |
-5.4 |
TP53 |
-5 |
| TNF-α |
-8.6 |
TNF-α |
-7 |
| Kaempferol-TNF-α |
|
Quercetin - TNF -α |
|
Discussion
Analysis on the health care mechanism of Crataegi fructus in
hypoglycemic and lipid-lowering
Literature retrieval and KEGG pathway enrichment analysis
showed that the health care effect of Crataegi fructus in hypoglycemic and lipid-lowering mainly involved PI3K/AKT, AMPK, TNF,
HIF-1, Insulin resistance and other signaling pathways, which were
the classical pathways for the occurrence and development of hyperlipidemia and hyperglycemia-related diseases, as well as the
important pathway for insulin to regulate lipid, glucose and lipid
metabolism. Under physiological conditions, secreted insulin activates the PI3K/AKT signaling pathway and regulates the balance
of lipid and glucose metabolism by increasing glucose utilization
and reducing gluconeogenesis in liver and muscle, and increasing
insulin production in pancreas [17]. HIF-1 in the HIF-1 signaling
pathway is a transcription factor that regulates cell response in
a hypoxic environment and plays a key role in the regulation of peroxisome lipid metabolism [18]. HIF-1 activates the expression
of visfatin 1 in liver tissues, resulting in the reduction of reactive
oxygen species and prevention of liver lipid deposition [19]. Studies have found [20] that in the process of inducing atherosclerosis, inflammatory cytokines, such as VEGF and TNF-α, will induce
the formation of vascular thrombosis and lead to a large number
of normal apoptosis to aggravate the development of the disease
in hyperlipidemia. Clinically, inflammatory factors such as IL6 and
TNF-α in the TNF signaling pathway have become important indicators to measure the development of hyperlipidemia, and TNF-α
and IL 6 can further accelerate the synthesis of Triglyceride(TG) by
stimulating the liver [21,22]. Insulin resistance is closely related
to hyperlipidemia [23]. Patients with hyperlipidemia are prone to
Insulin resistance. At the same time, insulin resistance makes insulin target cells insensitive to insulin, and plasma glucose cannot
be ingested by target cells, resulting in an increase in plasma glucose level. The body needs to ingest excessive glucose in plasma
to synthesize fat, which is stored in target cells, to maintain the
glucose metabolism balance, thus causing hyperlipidemia. AMPK
can prevent the occurrence and development of diabetes and
hyperlipidemia-related diseases by regulating glycolipid metabolism, anti-inflammation, and anti-oxidative stress [24]. Therefore,
Crataegi fructus can exert the effects of regulating apoptosis, inflammation, insulin level and immune function through multiple
signaling pathways such as PI3K/Akt, TNF, HIF-1, AMPK, Insulin resistance, in order to deal with the glucolipid metabolism disorder
caused by insulin level imbalance, immune function decline and
verification.
Analysis of key components of Crataegi fructus for hypoglycemic and lipid-lowering
Among 82 components of Crataegi fructus, 58 components
have action targets, among which 50 components have potential
contribution to the health care effect of hypoglycemic and lipidlowering. The topology analysis of component-target network revealed that the degree values of quercetin, kaempferol, ursolic
acid and epicatechin were the largest, suggesting that they had
more action targets, and might be important pharmacodynamics
substances for reducing blood glucose and regulating lipid of Crataegi fructus.
Studies have shown that quercetin has a variety of physiological activities, such as antioxidant, lipid-lowering, hypoglycemic, anti-inflammatory and so on. Ahn et al. [25] confirmed that
quercetin can exert its anti-adipogenesis activity by activating
AMPK signaling pathway. It has been proved [26] that quercetin
can significantly reduce IL6 and TNFα in high-fat and obese rats.
Quercetin indirectly affects PI3K/ AKT pathway by regulating ROS,
thereby inhibiting inflammation and apoptosis, and ultimately
reducing the degree of atherosclerosis. Quercetin can effectively
improve hyperglycemia, hyperlipidemia and antioxidant status in
type 2 diabetes [27] by influencing insulin system bypass through
AMPK pathway to alleviate insulin resistance [28]. Nutrients in
the diet, especially functional foods, are beneficial to metabolicrelated diseases such as diabetes. Kaempferol, as a dietary polyphenol [29], is reported to have a variety of beneficial effects on
human health, including the regulation of lipid and glucose metabolism, through the activation of AMPK to promote lipid metabolism [30] and up-regulation of skeletal muscle PI3K-AKT signaling
pathway [31], thereby improving glucose and lipid metabolism disorders and insulin resistance. In addition, kaempferol can also
play a role in reducing TG by inhibiting the Akt pathway [32]. Ursolic acid, as a natural Chinese herbal medicine component, can
effectively regulate the expression of pro-inflammatory or antiinflammatory cytokines such as TNFα and IL6 [33,34], which may
become an important target for the prevention and treatment of
inflammation and other inflammation-induced related diseases.
Ursolic acid can also inhibit 3T3-L1 pre-adipocyte differentiation
and adipogenesis through APMK pathway [35], thereby inhibiting 3T3-L1 pre-adipocyte differentiation and lipid accumulation
by regulating transcription factors and their downstream lipid
targets. The mechanism of ursolic acid in relieving insulin resistance in adipose tissues of aged rats is related to the activation
of Akt signaling pathway and inhibition of inflammation. Studies
in the literature [36,37] have shown that epicatechin can reduce
body weight, blood lipid and blood glucose of high-fat diet fed rat
model. Long-term administration of catechin can reduce obesity
in mice caused by high-fat diet, which may be related to the reduction of fat absorption and promotion of lipolysis. In addition,
epicatechin can improve insulin resistance in spontaneous type
II diabetes rats [38], which may be related to its effect in inhibiting liver gluconeogenesis. Epicatechin can also compensate for
insulin, promote adipocyte differentiation, and enhance insulin
sensitivity, which is beneficial to the prevention and treatment of
type II diabetes. The molecular docking results are also basically
consistent with previous studies, which provide certain evidence
support for the prediction results of this time. However, there
were few studies on INSR targets and HIF-1 pathway by quercetin,
kaempferol, ursolic acid and epicatechin, and few studies on the
mechanism of epicatechin on glucose and lipid reduction were
conducted, which could provide new ideas for the in-depth study
of glucose and lipid reduction in Crataegi fructus and worthy of
further exploration.
Conclusion
In the present study, network pharmacology was proposed
to systematically explore the substance basis, action target and
pathway information of Crataegi fructus for hypoglycemic and lipid-lowering. The results showed that the potential active components such as quercetin, kaempferol, ursolic acid, and epicatechin
in Crataegi fructus played key regulatory effects on apoptosis, insulin level imbalance, inflammatory response, and immune function caused by glycolipid metabolism disorder through PI3K/Akt,
AMPK, TNF and other signaling pathways. The predicted results
can provide theoretical basis and new research direction for the
pharmacodynamic substance basis and mechanism of Crataegi
fructu s hypoglycemic and lipid-lowering, and then provide new
reference for the app lication of Crataegi fructus and the development of health function food.
Declarations
Conflicts of interest: The authors declare no conflicts of interest.
Acknowledgments: This work was carried out with the support
of the Backbone Teachers Program of Sanquan College of Xinxiang Medical University (grant/award number: SQ 2021GGJS05)
and Science and Technology Public Relations of Henan Province
(grant/award number: 212102110187).
References
- National Pharmacopoeia Commission. Pharmacopoeia of the
People’s Republic of China. B eijing : China Medical Science and
Technology Press, 2015.
- Zhang Q J Y, Zhao P Y, Sun J, et al. Research progress on chemical
composition and pharmacological effects of Crataegi fructus. Northwest Pharmaceutical Journal. 2021; 36: 521-523.
- Aierken A, Buchholz T, Chen C, et al. Hypoglycemic effect of Crataegi fructus in type II di abetes mellitus rat model. Journal of the
science of food and agriculture. 2017; 97: 45574561.
- Zhang M. A Study on Hypolipidemic Effect of Crataegi fructus Flavonoids Extract. Medic inal Plant. 2017; 8: 45-47.
- Shih CC, Chen MH, Lin CH. Validation of the Antidiabetic and Hypolipidemic Effects of Clitocybe nuda by Assessment of Glucose
Transporter 4 and Gluconeogenesis and AM PK Phosphorylation
in Streptozotocin-Induced Mice. Evidence-Based Complementray
and A lternative Medicine. 2014; 2014: 705636.
- Shao F, Gu L, Chen H, et al. Evaluation of hypolipidemic and antioxidant effects in pheno l-rich fraction of Crataegus pinnatifida fruit in
hyperlipidemia rats and identification of che mical composition by
ultra-performance liquid chromatography coupled with
quadropole time-of-flight mass spectrometry. Pharmacognosy
Magazine. 2017; 13.
- Shao F, Gu L, Chen H, Liu R, Huang H, et al. Comparation of hypolipidemic and antioxidant effects of aq ueous and ethanol extracts
of Crataegus pinnatifida fruit in high-fat emulsion-induced hype
rlipidemia rats. Pharmacognosy Magazine. 2016; 12: 64-69.
- Zhang YQ, Li S. Some advances in modern research of network
pharmacology and tradit ional Chinese medicine. Chinese Journal
of Pharmacology and Toxicology. 2015; 29: 883-892.
- Xue RC, Fang Z, Zhang M, Yi Z, Wen C, et al. TCMID: Traditional
Chinese Medicine integrative database for herb molecular mechanism analysis. Nucleic acids research. 2013; 41: 1089-1095.
- Mandric I, Hill BL, Freund MK, Thompson M, Halperin E, et al. BATMAN: Fast and Accurate Integration of Single-Cell RNA-Seq Datasets via Minimum-Weight Matching. IScience. 2020; 23: 101185.
- Ru J, Li P, Wang J, Zhou W, Li B et al. TCMSP: a database of systems
pharmacology for drug discovery from herbal medicines. J Cheminform. 2014; 6: 1-6.
- UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019; 47: 506-515.
- Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, et al. The
GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Current Protocols in Bioinformatics. 2016; 54:
1-30.
- Rabadán R, Mohamedi Y, Rubin U, Chu T, Alghalith AN et al. Identification of relevant genetic alterations in cancer using topological
data analysis. Nature Communications. 2020 ; 11 : 3808.
- Liu S, X Hu, X Fan, Jin R, Yang W, et al. A Bioinformatics Research
on Novel Mechanism of Compoun d Kushen Injection for Treating
Breast Cancer by Network Pharmacology and Molecular D ocking
Verification. Evidence-based Complementary and Alternative Medicine. 2020; 2020: 1-14.
- Kft A, Mk A, Msa B, Shawky E. Identifying cancer-related molecular
targets of Nandina dome stica Thunb. by network pharmacology based analysis in combination with chemical profili ng and molecular docking studies. Journal of ethnopharmacology. 249:112413.
- Huang XJ, Liu GH, Guo J, Su Z. The PI3K/AKT pathway in obesity and
type 2 diabetes. International journal of biological sciences. 2018;
14: 1483-1496.
- Shi X, Sung S, Lee M, Kwok RTK, Herman HY, et al. A lipophilic
AIEgen for lipid droplet imaging and evaluation of the efficacy of
HIF-1 targeting drugs. Journal of Materials Chemistry B. 2020; 8:
1516-1523.
- Takatomo Arai, Masako Tanaka, Nobuhito Goda. HIF-1-dependent
lipin1 induction prevents e xcessive lipid accumulation in choline-deficient diet-induced fatty liver. Scientific Reports. 2018; 8:
14230.
- Hyun JA, Jung YK, Mi GG, Gu H, Kim HJ, et al. Beneficial Effects of
SREBP Decoy Oligodeoxynuc leotide in an Animal Model of Hyperlipidemia. International Journal of Molecular Sciences. 2020; 21:
552.
- Li H Y, Xi Qiaoyun, Zhang Yongliang. Research progress of TNF-α in
fat. China Animal Physiology and Biochemistry Academic Exchange
Conference. 2012.
- Zheng N, Xie Y M, Yu Hai, et al. Research progress of TNF-α and
obesity-related insulin resistance. Integrative Medicine Research.
2012; 4: 199-202.
- Chen JG. Hyperlipidemia and insulin resistance. Qinghai Med J.
2000; 030: 17-18.
- Ahn J, Lee H, Kim S, Park J, Ha T, et al. The anti-obesity effect of
quercetin is mediated by the AMP K and MAPK signaling pathways.
Biochemical and Biophysical Research Communications. 2008;
373: 545-549.
- Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M et al. Quercetin ameliorates metabolic syndrome and imp roves the inflammatory status in obese Zucker rats. Obesity. 2012; 16: 2081-2087.
- Lu XL, Zhao CH, Yao XL, Zhang H. Quercetin attenuates high fructose feeding-induced at herosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT sign aling pathway.
Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 2016; 85: 658.
- Soo-Mi, Jeong, Min-Jung, Choi HN, Kim JH, Kim JI. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant
status in type 2 diabetic db/db mice. Nutrition research and practice. 2012; 6: 201-207.
- Dhanya R, Arya AD, Nisha P, Jayamurthy P. Quercetin, a Lead Compound against Type 2 Diabet es Ameliorates Glucose Uptake via
AMPK Pathway in Skeletal Muscle Cell Line. Frontier s in Pharmacology. 2017; 8.
- Ochiai A, Othman MB, Sakamoto K. Kaempferol ameliorates symptoms of metabolic syndro me by improving blood lipid profile and
glucose tolerance. Bioscience, biotechnology, and biochemistry.
2021; 85: 2169-2176.
- Gao J, Zhang M, Niu R, Gu X, Erwei H et al. The combination of
cinnamaldehyde and kaempferol ameli orates glucose and lipid
metabolism disorders by enhancing lipid metabolism via AMPK
activation. Journal of Functional Foods. 2021; 83: 104556.
- Hoang M, Jia Y, Ji H L, Kim Y, Lee SJ. Kaempferol reduces hepatic
triglyceride accumulation by inhibiting Akt. Journal of Food Biochemistry. 2019; 43: 13034.
- Zhang Z, Sun W, Liu TH. Effect of kaempferol on PI3K-AKT-GLUT4 signaling pathway in skeletal muscle of type 2 diabetic mice. Proceedings of the 4th Experimental Medicine Professional Committee of
Chinese Association of Integrated Traditional and Wes tern Medicine. 2016.
- Zeng Lu, Tang W J, Yin Jinjin. Experimental study on ursolic acid
in hepatocyte steatosis. Chinese Journal of Medicinal Materials.
2015; 38: 1049-1052.
- Qi MY, Yang JJ, Zhou B. Effects of ursolic acid on diabetic nephropathy in mice. Chinese Journal of Applied Physiology. 2014; 445-448.
- Ding QC, Feng LY, Ying N. Ursolic acid activates autophagy through
AMPK med iated signaling pathway to improve oleic acid-induced
lipid deposition in hepatocytes. Journ al of Zhejiang Traditional
Chinese Medicine University. 2019; 043: 1150-1155.
- Gu FF, SUN Y, XU G. Effect of catechins on metabolic syndrome in
nutritionally obese rats. Journal of practical medicine. 2010; 27:
830-832.
- Hou HM, Yang WL, Bao SQ. Epigallocatechin gallate inhibits TLR4
inflammator y pathway and insulin resistance in obese rats.
Chinese journal of histochemistry and cytochemistry. 2019; 28:
204-210.
- Zhao XZ, Qiao WW. Effects of epigallocatechin gallate on insulin
resistance in rats. Chi nese journal of experimental animal science.
2011; 19: 489-494.