Introduction
Brenner tumors constitute 1-2% of all primary ovarian tumors.
According to the WHO classification they are divided into the following categories: a) benign Brenner tumors b) Brenner tumors
of borderline malignancy and c) malignant Brenner tumors. The
benign counterparts account for 4-5% of all benign ovarian tumors from the surface epithelium [1]. In the majority of the cases
(more than 50%) the size of the tumor is less than 2 cm and is discovered incidentally during the macroscopic examination of the
ovaries for other causes [2-5]. Although the size of the malignant
and of the low malignant potential tumors characteristically ranging from 10 to 30 cm., in the benign forms only in 10% of the cases
the size is bigger than 10 cm [6].
We present a case of an 18 cm benign Brenner tumor in a
59-year-old woman.
This represents a case of unusually large Brenner tumor which
can be considered one of the largest ever documented in the literature.
Case report
Our case refers to a 59-year-old woman who presented with
uterine bleeding. Clinically an abdominal mass was palpable.
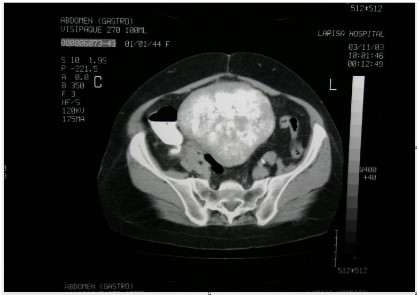
A computed tomographic (CT) scan displayed a left solid adnexal mass.
Surgical excision of the mass and total hysterectomy was performed.
Gross features
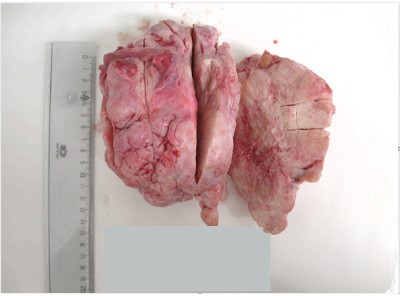
We received a 18 × 13,5 × 12 cm tumor for frozen sections examination.
Grossly, the tumor had lobulated outline and solid, firm, gray-white, fibromatous cut surface. The frozen sections were negative
for malignancy.
Leiomyomas and adenomatous endometrial polyp were identified in the uterus.
Microscopic findings
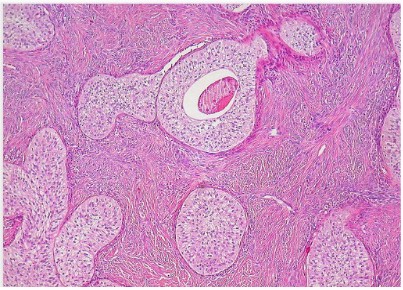
Histological examination of multiple sections showed the features of typical benign Brenner tumor characterized by sharply
demarcated nests of epithelial cells within an abundant fibrous
stroma. Some epithelial islands exhibited central lumens with eosinophil material. The epithelial cells were polygonal with eosinophil or clear cytoplasm. The nuclei were of similar size, oval with
obvious nucleolus and longitudinal grooves.
The mitosis were rare and cellular atypia wasn’t observed.
Hyalinization, extensive calcification and stromal luteinization
were also observed.
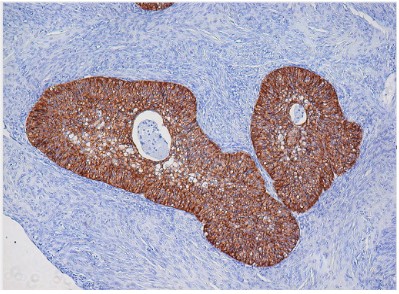
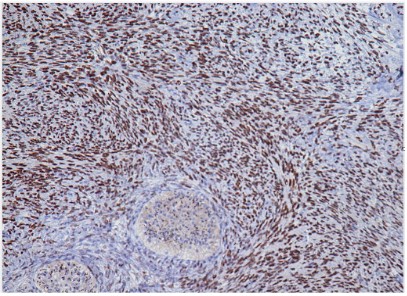
The immunohistochemical examination showed that the epithelial cells were positive for the cytokeratin 7 while they were
negative for the cytokeratin 20.
The stromal cells were positive for the progesterone receptors
but negative for the estrogen receptors.
Discussion and conclusion
Brenner tumor was first described by Fritz Brenner in 1907 [7].
The average age at presentation is about 50 years, with 71% of
the patients being over 40 years of age [2,3]. Most of the patients
are asymptomatic and the neoplasm is usually an incidental finding during operation for other gynecological reasons or the macroscopic examination of the ovaries for other causes [2-5]. Non-specific signs and symptoms have been mentioned when the size
of the tumor is large. They occasionally are associated with manifestations related to the elaboration of estrogens or androgens by
the stroma of the tumor. Rare extraovarian Brenner tumors are
reported [8-10].
Grossly, these tumors vary greatly in size, are solid with lobulated outline and white or yellowish white. They usually are less
than 2 cm., unilateral, frequently located in the right ovary. Approximately 6% are bilateral. A 10% to 25% of the cases appear
as small, firm nodules in the wall of a mucinous cystadenoma.
Occasionally th ey are associated with a dermoid cyst and rarely
with carcinoid or struma ovarii [11]. Occasionally, the tissue is
gritty, owing to calcific deposits, and rarely it is massively calcified.
Rarely benign Brenner tumors are large and predominantly cystic. In contrast borderline tumors are characteristically cystic and
unilocular or multilocular, with papillomatous masses protruding
into cystic cavities. Malignant Brenner tumors may be solid or
cystic and in contrast to transitional cell carcinomas, may have a
variably sized component that resembles benign Brenner tumor.
Microscopically, benign Brenner tumors composed of rounded, sharply demarcated nests of epithelial cells lying within an
abundant fibromatous stroma. The nests may be solid or have a
central lumen that contains dense eosinophilic material or mucin.
The epithelial cells resemble transitional epithelium (urothelium), are polygonal to ovoid and have pale cytoplasm and oval
nuclei with obvious nucleolus and in some of them longitudinal
grooves. The cells lining the lumens range from flat to columnar,
often contain mucin. Cysts of varying sizes lined entirely by mucinous or transitional epithelium are sometimes encountered. Dystrophic calcinosis and stroma edema is observed in a percentage
of 50% of the cases, like in our case [11].
The diagnosis is based on the above typical morphologic findings.
The term “metaplastic Brenner tumor” is suggested in the cases that crowed transitional nests and cysts with mucinous component exist, because the epithelial elements are mixed and not
separated [12,13].
The differential diagnosis, especially when the size of the tumor is large, must be done from the low malignant potential tumors and malignant Brenner tumors. The gross findings and the
examination of multiple sections is necessary in these cases.
The histogenesis of the Brenner tumors has provoked considerable debate. Origin from granulose cells, the rete ovarii, the
Walthard nests, teratomas of the ovary or the stromal cells has
been proposed. However, nowadays it is accepted that derive
from epithelium of the ovarian cortex through transitional metaplasia [14-17].
Immunohistochemically, the positivity of the benign Brenner
tumors for Uroplakin III suggests urothelial differentiation, a finding which is absent in the majority of the low malignant potential tumors and the malignant forms [15-17]. The neoplastic cells
are negative to the Thrombomodulin while the positivity to the
keratin 20 varies among various studies [18,21,22]. In our case
positivity to the keratin 7 was observed while the keratin 20 was
negative.
The studies with the electron microscope were refutable. An
older study held by Shevchuk and Associates supports that the
cells towards the center of the solid groups present findings
which are similar to the intermediate urothelial layers 19. However, a recent study held by Ordonez and associates objects to the
above findings [14].
In summary, the greater size of Brenner tumors has been
thought to be a predictor of malignancy. However, it is possible
for larger lesions to have benign morphology and behavior. Multiple cut sections must be examined to exclude malignancy and to
confirm the benign behavior.
References
- Nakashima N, Nagasaka T, Fukata S, Oiwa N, Nara Y, Fukatsu
T, Takeuchi J. Study of of ovarian tumors treated at Nagoya
University Hospital 1965-1988. Gynecology Oncology. 1990;
37: 103-111.
- Ehrlich CE, Roth LM. The Brenner tumor. A clinicopathologic
study of 57 cases. Cancer. 1971; 27: 332-342.
- Fox H, Agrawal K, Langley FA. The Brenner tumor of the ovary A clinicopathologic study of 54 cases. J Obstet Gynaecol
Br Commonw. 1972; 79: 661- 665.
- Silverberg SG. Brenner tumor of the ovary. A clinicopathologic study of 60 tumors in 54 women. Cancer. 1971; 28:
588-596.
- Waxman M. Pure and mixed Brenner tumors of the ovary:
clinicopathologic and histogenetic observations. Cancer.
1979; 43: 1830-1839.
- Tabassoli FA, Devilee P. (Eds): World Health Organization of
Tumors. Pathology and Genetics of Tumours of the Breast
and Female genital Organs. IARC Press: Lyon 2003.
- Brenner F. Das Oophoroma Folliculare. Frankfurt Z. Pathol.
1907; 1: 150-171
- Hu RY, Deng YJ, Zhu HH, Zhou J, Hu M, et al. Extraovarian
Brenner tumor in the uterus: a case report and review of
literature. Diagn Pathol. 2020; 15: 22.
- Hwang CS, Lee CH, Lee SJ, Kim YG, Kim A, et al. A peculiar
case report of extraovarian Brenner tumor arising in the
omentum. World J Surg Oncol. 2017; 15: 72.
- Shaco-Levy R, Benharroch D, Shaco-Levy R, et al. Vaginal
brenner tumor. Int J Gynecol Pathol. 2013; 32: 238-241.
- Scully RE, Young RH, Clement PB. Tumors of the ovary maldeveloped gonads, fallopian tube and broad ligament. In:
Atlas of tumor pathology, 3rd series, fascicle 23. washington
D.C. Armed Forces Institute of Pathology, 1998.
- Roth LM, Dallenbach – Hellweg G, Czernobilsky B. Ovarian
Brenner tumor, I: Metaplastic, proliferating, and of low malignant potential. Cancer. 1985; 56: 582-591.
- Roth LM, Langley FA, Fox H, Wheeler JE, Czernobilsky B.
Ovarian clear cell adenofibromatous tumors: Benign, of low
malignant potential, and associated with invasive clear cell
carcinoma. Cancer. 1984; 53: 1156-1163.
- Αrey LB. The origin and form of Brenner tumor. Am J Obstet
Gynecol. 1961; 81: 743-751.
- Merkow LP, Salazar H, Pardo M. Human ovarian neoplasms:
light and electron microscopic correlation, I: Brenner tumours. Obstet Gynecol. 1972; 40: 667-680.
- Shevchuk MM, Fenoglio CM, Richart RM. Histogenesis of
Brenner tumors: II. Histochemistry and CEA. Cancer. 1980;
46: 2617-2622.
- Τakeuchi K, Ohbayashi C, Kitazawa S, Ohara N, Maruo T. Co-existence of Brenner tumor and struma ovarii: case report.
Eur J Gynaecol Oncol. 2005; 26: 109-110.
- Riedel I, Czernobilsky B, Lifschitz-Mercer B, Roth LM, Wu
XR, et al. Brenner tumor but not transitional cell carcinomas
of the ovary show urothelial differentiation: immunohisto-chemical staining of urothelial markers, including cytokeratins and uroplakins. Virchows Arch. 2001; 438: 181-191.
- Ogawa K, Johansson SL, Cohen SM. Immunohistochemical
analysis of uroplakins, urothelial specific proteins in ovarian
Brenner tumors, normal tissues, and benign and neoplastic
lesions of the female genital tract. Am J Pathol. 1999; 155:
1047-1050.
- Logani S, Oliva E, Amin MB. Immunoprofile of ovarian tumors with putative transitional cell (urothelial) differentiation using novel urothelial markers. Histogenetic and diagnostic implications. Am J Surg Pathol. 2003; 27: 1434-1441.
- Ordonez NG, Mackay B. Brenner tumor of the ovary: a comparative immunohistochemical and ultrastructural study
with transitional cell carcinoma of the bladder. Ultrastruct
Path. 2000; 24: 157-167.
- Ordonez NG. Transitional cell carcinomas of the ovary and
bladder are immunophenotypically different. Histopathology. 2000; 36: 433-438.