Introduction
The link between onco-hematologic diseases and oxidative
stress has been reviewed where continued oxidative stress activated genes involved in growth factors, inflammatory cytokines,
cell cycle regulatory molecules and anti-inflammatory molecules
therefore leading to cancer [1]. Oxidative stress is a prominent
feature of both acute and chronic diseases and cancer including
leukemias [2]. Benzene is a widely utilized solvent but constitutes
an occupational hazard as it caused adverse health effects such as
acute myelogenous leukemia [3]. However, the mechanism employed in benzene’s toxicity that led to acute myeloid leukemia
and myelodysplastic syndromes remains largely unclarified [4].
Hematological toxicity by benzene may be linked to its active
metabolite such as benzoquinone that induces DNA strand breaks
[5]. The bioactivation of benzene resulted in increase in oxidative
stress that plays key roles in its cellular toxicity. Benzene is metabolized into hydroquinone and catechol in liver which are further
converted by myeloperoxidase to 1,4-benzoquinone and 1,2-benzoquinone, respectively in the bone marrow [6]. Many of metabolites of Benzene have been associated with increase myeloid
cell growth in vitro by the formation of reactive oxygen species
ROS [7].
The interest on medicinal herb is due to its secondary metabolites that possess medicinal properties and its essentiality in pharmaceutical development [8]. Eulophia gracilis is one of several
species of orchids with medicinal utility for treatment of diabetes,
cancer, blood diseases, sexual impotency and male sterility by traditional healers in Nigeria especially among indigenous people in
Oyo town and Jigawa in Nigeria. The plant has been shown to be
rich in phytochemicals like glycoside, alkaloids, tannins, phlobatanins and flavonoids which are of medicinal values [9]. Many of
these phytochemicals have been extensively studied to possess
antioxidant capacity that are of pharmacological importance in
decreasing the harmful effects of “reactive species”, such as reactive oxygen and nitrogen molecules on a cellular level in humans.
It is therefore on this note, that the present study investigated the
anti-leukemogenic and myeloprotective effect of Eulophia gracilis
on Benzene-induced hematological perturbation.
Materials and methods
Chemicals and Reagents
Reduced glutathione, 5’,5’-dithio-bis-2-nitrobenzoic acid, thiobarbituric acid, epinephrine, Potassium iodide, 1-chloro-2, 4-dinitrobenzene and hydrogen peroxide were purchased from Sigma®
Chemical Company (London, UK).
Plant collection, identification and preparation
Fresh Eulophia gracilis plants were collected from rocky area
in Oyo, Nigeria. The plant was identified and authenticated by
plant taxonomist at Herbarium section of Department of Botany,
University of Ibadan, Nigeria with University of Ibadan Herbarium
(UIH) number 22528. The tubers were thoroughly washed with
tap water, sliced into pieces and shed dried. The dried tubers
were pulverized into powder form using electric grinder. 200g of
Eulophia gracilis fine powdered sample was extracted with 800
mL aqueous methanol (20:80) for 48 hours by cold marceration.
The extract was evaporated under reduced pressure by using a
rotary evaporator and further lyophilized using freeze-dryer machine into powdery marc tagged AMEG (aqueous methanolic extract of Eulophia gracilis).
Experimental animals
Male wistar rats of weight range 100 g - 120 g were used for
this study. The animals were got from McTemmy farm and acclimatized for two weeks in the animal house of the Department
of Chemical Sciences, Ajayi Crowther University, Oyo. They were
kept in a well ventilated research cages and fed with standard
commercial rat feed (Ladokun feeds Nigeria Ltd.) and clean tap
water supplied ad libitum. Anesthesia was not involved in this research and the protocol conformed to the guidelines of the National Institute of Health for laboratory animal care and use [10].
Treatments and animal grouping
After adaptation period, the animals were randomly assigned
into seven main experimental groups of 6 animals each. The rats
were intravenously administered with 175 mg/kgbw of benzene
(in water; 2-propanol, 1:1) every other day for 5 consecutive
weeks and this successively initiated leukemogenesis in the rats.
The doses of the extract was carefully chosen from pilot study
and that of cyclophosphamide were chosen from literature which
were administered once daily and orally as shown in the table 1.
Collection of blood and liver samples
24 hours after the final treatment, the blood samples were collected from each animal through retro orbitals plexus into lithium
heparinized tubes and thereafter sacrificed by cervical dislocation. The bone marrow from femurs was collected for histopathological examination.
Measurement of haematological parameters
Haemoglobin concentration, % packed cell volume (PCV), red
blood cell (RBC) count, haemoglobin concentration, white blood cell (WBC) count, % Neutrophills, % Lymphocytes and platelets
count were determined using the automated blood analyser, SYS-
MEX KX21.
Table 1: Experimental design.
| Treatment groups |
Treatment |
| A. Control |
Distilled water |
| B. Leuk untreated (leukemia-initiated rats without extract treatment) |
175 mg/kgbw benzene mixture every two days for 5 weeks |
| C. Leuk + AMEG Post-treated |
Leukemia induction then followed by 2 weeks of 200 mg/kg AMEG treatment |
| D. Leuk + AMEG + CYP Post-treated |
Leukemia induction then followed by 2 weeks of 200 mg/kg AMEG and 2 mg/kg
cyclophosphamide treatment |
| E. Leuk + CYP Post-treated |
Leukemia induction then followed by 2 weeks of 2 mg/kg cyclophosphamide treatment |
Assay for oxidative stress maker in the plasma and bone marrow gene expression
AOPP in plasma was estimated by the method described by
Witko et al. [11] as modified by Zhang et al. [12]. Plasma total
thiol were measured spectrophotometrically using DTNB (2, 2’-dinitro-5, 5’-dithiodibenzoic acid) [13]. Protein carbonyl content in
liver was determined according to the procedure of Reznick and
Packer [14] where 2,4-dinitrophenylhydrazine (DNPH) reacts with
protein carbonyls, forming a Schiff base to produce the corresponding hydrazone. The amount of protein–hydrazone produced
is quantified spectrophotometrically at 370 nm. The expression
of gene products of p53, p38, inducible nitric oxide synthase, IL-6
and CD79 in rat bone marrow were determined by immunohistochemistry.
Results
Hematological parameters of control, post leukemia induction, and after treatment with extract of Eulophia gracilis (mean
± SD)
The protective effect of Eulophia gracilis on hematological perturbation by benzene was presented in table 2. PCV and platelets
were drastically reduced in leukemic rats when compared with
control group. However, white blood cells were greatly increased
in rats treated with benzene when compared with control group.
Moreover, anisocytosis, Poikilocytosis and formation of up to 4%
blasts were observed in blood film of leukemic rats when compared with control group. However, post-treament of leukemia-induced rats with aqueous methanolic extract of Eulophia gracilis
protected against influence of benzene on these hematological
parameters and caused a disappearance of blast in the blood film.
Table 2: Hematological parameters of control, post leukemia induction, and after treatment with extract of Eulophia gracilis (mean ± SD).
| GROUP |
PCV |
HGB |
RBC |
WBC x103/μL |
platelet |
Anisocytosis |
Poikilocytosis |
Percentage blast |
| Ctrl |
50.5 ± 6.66 |
11.74 ± 0.83 |
6.65 ± 1.20 |
4500.00 ± 711.81 |
102666.7 ± 8326.664 |
Negative |
Negative |
0 |
| Leuk untreated |
40.75 ± 3.40 |
6.85 ± 0.57 |
4.09 ± 0.65 |
7250 ± 1138.71 |
248666.67 ± 55967.25 |
Positive |
Positive |
4 |
| Leuk + EG Post-treated |
42.33 ± 3.21 |
9.65 ± 0.28 |
6.25 ± 0.64 |
4933.33 ± 945.16 |
184666.7 ± 40451.62 |
Negative |
Negative |
0 |
| Leuk + EG + CYP Post-treated |
45.25 ±4.11 |
10.60 ± 1.30 |
5.94 ± 0.66 |
6075 ± 411.3 |
159666.7 ± 16653.33 |
Negative |
Negative |
0 |
| Leuk + EG + CYP Post-treated |
45.25 ±4.11 |
10.60 ± 1.30 |
5.94 ± 0.66 |
6075 ± 411.3 |
159666.7 ± 16653.33 |
Negative |
Negative |
0 |
Abbreviations: PCV: Packed Cell Volume; LYM: Lymphocytes; WBC: White Blood Cell.
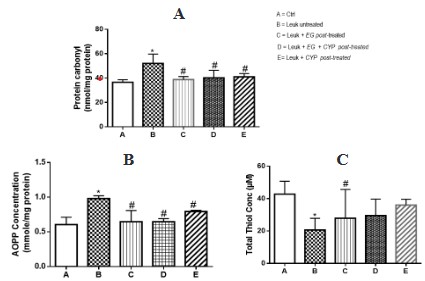
Effect of Eulophia gracilis extract on Benzene – induced oxidative stress in the plasma of leukemia bearing rats
The protective effect of aqueous methanolic extract of Eulophia gracilis on Benzene – induced Oxidative Stress in the plasma
of Leukemia bearing rats is shown in figure 1. The intravenous
administration of Benzene to rats resulted into a significant elevation in the concentration of advanced oxidation protein products
(AOPPs) and protein carbonyl content present in the plasma of rats by 62.13% when compared with the control group (Figure 1A
and B). There was also a concormitant significant reduction in total thiol content by 51.84% when compared to the control group
(Figure 1C). However, post-treatment with aqueous methanolic
extract of Eulophia gracilis significantly (p < 0.05) attenuated the
effect of Benzene toxicity by significantly reducing the generation
of plasma AOPP and protein carbonyl and restore sulfhydryl level
when compared to animal group exclusively treated with Benzene.
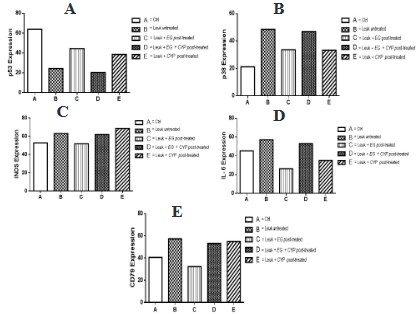
Influence of Eulophia gracilis extract on p53, p38, iNOS, IL-6
and CD79 expression in bone marrow cell of benzene-induced
leukemic rats
The results of Influence of Eulophia gracilis Extract on p53,
p38, iNOS, IL-6 and CD79 expression in bone marrow cell of Benzene-induced Leukemic rats were presented in figure 3.
The expression of tumor suppressor protein p53 was low in
leukemic rats relative to control animal group (figure 3A). However, treatment of rats with plant extract before and after leukemia induction upregulated the expression of p53 gene relative
to untreated leukemic rats. There was an increase in expression
of stress kinase p38, inducible nitric oxide (iNOS), proinflammatory cytokine IL-6 and CD79 gene proteins in leukemogenesis-initiated rats in figure 3B, C, D and E respectively when compared
with control rats. However, rats co-treated and post-treated with
AMEG showed lower expression of the stress kinase p38, iNOS,
IL-6 and CD79 proteins relative to the leukemic rats. Also, there
was upregulation of the p38 protein in animals post-treated with
both extract and cyclophosphamide which might probably due
to overload of the drug and the plant extract resulting in further
stress to the animals.
Discussion
The present work evaluated the potential of aqueous methanolic extract of Eulophia gracilis pseudobulbs against leukemogenic process initiated in Wistar. In this study, the significant increase in WBCs in leuk group relative to control group corroborated the leukocytosis that was earlier reported in leukemic rats and
humans [15]. This was ameliorated in rats treated with aqueous
methanolic extract of Eulophia gracilis before and after exposure
to the leukemic-inducing agent. An anisocytosis has been listed as
a morphological feature of the occurrence of acute panmyelosis
with myelofibrosis. The result showed a presence of anisocytosis and poikilocytosis in leuk group due to exposure to benzene
mixture. This result is in consonance with earlier finding of other
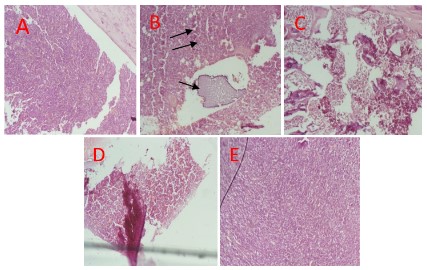
works [16]. Moreover, the presence of blast and hypercellulariy
observed in the peripheral blood and bone marrow of rats in leuk
group are notable laboratory picture of leukemia. However, these
hematological alternances were buffered when the rats were treated with AMEG extract.
Assessment of products of damaged protein and lipid contents
can be used as indirect quantification of reactive oxygen species
[17]. The sulfhydryl groups present on protein make up the major
plasma antioxidants in vivo and are mostly present on albumin
making it to be the reducing groups constituent in body fluids
[18]. Proteins has the capacity through thiol groups present on
them to scavenge 50% – 75% of reactive species generated [19].
Oxidation of critical sulfhydryl-containing proteins is also associated with depleted reduced glutathione which is a cellular nonprotein thiol defenses system [20]. The reduction in plasma levels of
protein thiol correlated positively with the increased levels of lipid
peroxides and advanced oxidation protein products (AOPPs) [21].
In this present work, the exposure of rats to benzene resulted in
declined plasma total thiol with concomitant increase in AOPPs
relative to control. The AOPPs are produced in plasma when the
plasma albumin is subjected to oxidation by various oxidants [22]
and its elevated levels has been associated with some pathological conditions such as atherosclerosis, diabetes, nephropathies
and cancer [23]. However, administration of extract of Eulophia
gracilis in this study significantly reduced the elevated generation of AOPP in leukemogenesis-initiated rats group and effectively restored the total thiol status when compared to untreated
group. This shows that the extract may improve plasma antioxidant activity by preserving sulfhydryl protein pools.
Elevated level of protein carbonyl in leukemia bearing rats in
this present work relative to control supports the hypothesis that
high ROS generation occurs in neoplastic or cancer cells. Protein
carbonyl is a product of irreversible non-enzymatic oxidation of
protein and serves as biomarker of disease progression in oxidative stress-mediated pathophysiologies [24]. Carbonylation of
protein often leads to a loss of protein function and therefore
marker of severe oxidatively damage and disease-derived protein
dysfunction [25]. However, administration of Eulophia gracilis
pseudobulb extract significantly reduced (p < 0.05) the levels of
protein carbonyl when compared to the untreated leukemia-bearing rats. This therefore suggested the antioxidant potential of
this plant extract.
The tumor suppressor p53 plays an important role in regulating hematopoietic stem cells (HSC) quiescence and self-renewal
during steady-state hematopoiesis [26] and its loss has been associated to decreased apoptosis and an increase in disease progression of leukemic cells and hematologic tumors [27]. In the present
study after intravenous exposure of rats to benzene for 4 weeks
consecutively, our results showed that the expression of p53
protein in benzene exposed animal group marrow cells declined
markedly relative to control animal group. It has been demonstrated that decreased levels of p53 in bone marrow may lead to reduction in apoptosis and increase in bone marrow cellularity [28].
This may be implicated in hypercellularity observed in bone marrow histomicrogram observed in this study. However, the post-treatment of animal group exposed to benzene with methanolic
extract of Eulophia gracilis offer protection by upregulating p53
protein expression in these animal bone marrow cells relative to
untreated animal group and therefore lead to moderate cellularity observed in the bone marrow cells of animal group treated
with the plant extract.
Benzene metabolites were shown to stimulate the production
of proinflammatory cytokines such as TNF-α and IL-6 by activated human peripheral blood mononuclear cells (PBMC) [29]. DNA
damage induced by Benzene metabolites and Ionizing radiation
have been shown to initiate the expression of various circulatory cytokines such as IL-6 and this response may be related to
apoptosis. IL-6 is a pluripotent cytokine found to be involved in
acute pro-inflammatory process associated with overexposure to
ionizing radiation and leukemogenic benzene intermediates [30].
Our result showed a high expression of IL-6 in the bone marrow
of rats exposed to benzene. This increase in IL-6 expression is in
agreement with findings of other researchers on animal exposed
to radiation which is also a leukemogenic factor and human peripheral blood mononuclear cells activated by benzene metabolites
[29]. However, post-treatment of exposed animals with extract of
Eulophia gracilis pseudobulb resulted into low positive expression
of this cytokine. This result suggested that the protection of these
rats from benzene-induced hematopoietic damage may also be
mediated by cytokines and chemokines.
The contribution of aromatic hydrocarbons in mediating inflammatory signaling through stress kinase p38 MAP Kinase that
influences production of various cytokines has been reported
[31]. Protein phosphorylation and dephosphorylation mediated
by protein kinase and phosphatase respectively is central to regulation of many cellular processes in biological system. Previous
findings implicated benzene and its metabolites as activator of
several key signaling pathways such as p38 and resultantly triggering apoptosis of cells of the marrow or malignant progression of
human leukemia cells [32] and therefore suggesting the involvement of protein kinase in benzene-induced toxicity.
The present work showed the elevated expression of p38
protein in the rats exposed to benzene relative to control group.
Benzene component of Tobacco smoke has been inferred to activate p38 in an oxidative stress dependent way [33]. Therefore,
increased expression of p38 protein in marrow cells of animals
exposed to benzene may be attributed to oxidative stress mediated by benzene or its metabolite. However, administration of Eulophia gracilis subsequent to leukemia induction down regulate
the p38 protein expression. This show that the extract may offer hematoprotective via p38 down regulation in oxidative stress
condition caused by environmental benzene pollutant.
Benzene has been shown to induces iNOS and generates nitric oxide and reactive oxygen species like hydroxyl radicals, superoxide anion (O2 •−), hydrogen peroxide and singlet oxygen in
the mice marrow cells [34]. The iNOS-deficient mice were reported to be partially protected from benzene induced bone marrow suppression and this supported a link between nitric oxide
production and hemotoxicity [35]. The result of this work presented an elevated expression of iNOS in the bone marrow of animal
exposed to benzene relative to control. However, administration
of the extract to animal group that were induced with leukemia
show relatively reduced expression of iNOS protein. More importantly, pre-treated or co-treated with extract show relatively more
effect in lowering expression of iNOS while co-administration of
extract and standard drug do not produce any noticeable effect.
This means that the extract may offer preventive effect on iNOS
induction by benzene intoxication.
CD79 is heterodimeric transmembrane protein that is associated with membrane immunoglobulin and expressed in B cell
at early stages of its development until the last stage of maturation before differentiation to plasma cells [36]. It has also been
found in biphenotypic leukemia cases of myeloid leukemia, where
it was coexpressed with myeloid markers on bone marrow blast
cells [37]. Prevous work showed that CD79a plays an important
functional role in maintaining the immature, immune suppressive
phenotype of myeloid-derived suppressor cells and in inducing
the secretion of protumorigenic cytokines. The result of this study
revealed the higher positive expression of CD79 protein in bone
marrow of animal group exposed to benzene relative to low positive expression in control group. This agree with the observation
of other researchers that benzene is a risk factor for development
of acute myeloid leukemia. However, in the animal group that
were post-treated with extract of Eulophia gracilis, the expression
of CD79 protein was reduced relative to the Leuk group. Therefore, it is suggested that the phytoactive principle in this extract
may downregulate the expression of CD79 in myeloid-derived
suppressor cells and prevent the induction of protumorigenic cytokine like interleukine-6 as observed in this study.
Overall, aqueous methanolic extract of Eulophia gracilis protected against the cellular and biomolecular oxidation in the
blood system and modulated the expression of genes involved in
biosignalling and regulation of myeloid proliferation. The effects
of this plant may be due to antioxidant capacity of inherent active
compounds.
Acknowledgements: We acknowledge the effort of Mr. Salawu
of Department of haematology, University College Hospital Ibadan who assisted in hematological analysis and blood morphological scoring.
References
- Imbesi S, Musolino C, Allegra A, Saija A, Morabito F, et al. Oxidative
stress in oncohematologic diseases: an update. Expert Rev Hematol. 2013; 6: 317-325.
- John D. Hayes, Albena T. Dinkova-Kostova and Kenneth D. Tew
2020 Oxidative Stress in Cancer Cancer Cell. 2020; 38: 167-197.
- Robert Snyder. Leukemia and Benzene. Int J Environ Res Public
Health. 2012; 9: 2875-2893.
- Roma-Torres J, Teixeira JP, Silva S, Laffon B, Cunha LM, et al. Evaluation of genotoxicity in a group of workers from apetroleum refinery aromatics plant. Mutat Res. 2006; 604: 19-27.
- Sze CC, Shi CY, Ong CN. Cytotoxicity and DNA strand breaks induced by benzene and its metabolites in Chinese hamster ovary
cells. J Appl Toxicol. 1996; 16: 259-264.
- Short DM, Lyon R, Watson DG, Barski OA, Garvie GM, et al. Metabolism of trans, trans-muconaldehyde, a cytotoxic metabolite
of benzene, in mouse liver by alcohol dehydrogenase Adh1 and
aldehyde reductase AKR1A4. Toxicology and Applied Pharmacology. 2006; 210: 163-170.
- Martyn T, Smith. The Mechanism of Benzene-induced Leukemia: A
Hypothesis and Speculations on the Causes of Leukemia. Environmental Health Perspectives. 1996; 104: 6.
- Bailly C. Ready for a comeback of natural products in oncology.
Biochemical Pharmacology. 2009; 77: 1447-1457.
- Ola OS. Preliminary Proximate Analysis, Chemical Composition and
Phytoconstituents of Eulophia gracilis Orchid. International Journal of Sciences: Basic and Applied Research. 2017; 36: 215-222.
- National Research Council. Guide for the care and use of laboratory animals, 8th ed. Washington, DC: National Research; The National Academies Press; 2011.
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa
T. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996; 49: 1304-1313.
- Zhang ZH, Jhaveri DJ, Marshall VM, Bauer DC, Edson J, et al. A comparative study of techniques for differential expression analysis on
RNA-Seq data. PLoS One. 2014; 9: e103207.
- Motchnik P, Frei B, Ames B. Measurement of antioxidants in human blood plasma. Methods Enzym. 1994; 234: 269-279.
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994; 233:
357-363.
- Akanni EO, Folarin OR, Igbeneghu C. Tumour Suppressive and Organ Protective Effects of Aqueous Andrographis paniculata Leaves
Extract on Benzene Induced Leukaemia Bearing Rats, Annual Research & Review in Biology. 2014; 4: 1070-1079.
- Keith A, Baarson Snyder CA, Green JD, Sellakumar A, Goldstein BD,
et al. The Hematotoxic Effects of Inhaled Benzene on Peripheral
Blood, Bone Marrow, and Spleen Cells Are Increased by Ingested
Ethanol, Toxicology and Applied Pharmacology. 1982; 64: 393-404.
- Sunaiba M, Avinash SS, Arunkumar K, Malathi M, Shivashankara
AR, et al. Analytical Method Comparison Of Advanced Oxidation
Protein Products (Aopp) With Modified Aopp, International Journal of Clinical Biochemistry and Research. 2015; 2: 5-12.
- Prakash M, Upadhya S, Prabhu R. Protein thiol oxidation and lipid
peroxidation in patients with uremia. Scand J Clin Lab Invest. 2004;
64: 599-604.
- Davies MJ, Fu S, Wang H, Dean RT. Stable markers of oxidant damage to proteins and their application in study of human diseases.
Free Radic Biol Med. 1999; 27: 1151-1161.
- Pascoe GA, Olafsdottir K, Reed DJ. Vitamin E protection against
chemical-induced cell injury. I. Maintenance of cellular protein
thiols as a cytoprotective mechanism. Arch. Biochem. Biophys.
1987; 256: 150-158.
- Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol
oxidation and carbonyl formation in chronic renal failure. Kidney
Int. 2000; 57: 2571-2578.
- Capeillere-Blandin C, Gausson V, Descamps-Latscha B, WitkoSarsat V. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochim Biophys Acta. 2004;
1689: 91-102.
- Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, Nguyen AT,
Gausson V, et al. Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am J Kidney Dis. 2005; 45: 39-47.
- Ray G, Husain SA. Oxidants, antioxidant and carccinogenesis. Ind J
Exp Biol. 2002; 40: 1213-1232.
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milazani A. Biomarkers of oxidative stress in human disease. Clin Chem. 2006; 52:
601-623.
- Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, et al. p53 Regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2005; 4: 37-48.
- Pant V, Quintás-Cardama A, Lozano G. The p53 pathway in hematopoiesis: lessons from mouse models, implications for humans.
Blood. 2012; 120: 5118-5127.
- Long DJ, Gaikwad A, Multani A, Pathak S, Montgomery CA, et al.
Disruption of the NAD(P)H:Quinone Oxidoreductase 1 (NQO1)
Gene in Mice Causes Myelogenous Hyperplasia. Cancer Res. 2002;
62: 3030-3036.
- Gillis B, Gavin IM, Arbieva Z, King ST, Jayaraman S, et al. Identification of human cell responses to benzene and benzene metabolites. Genomics. 2007; 90: 324-333.
- Petit-Frère C, Capulas E, Lyon D, Norbury C, Lowe J, et al. Apoptosis
and Cytokine Release Induced by Ionizing or Ultraviolet B Radiation in Primary and Immortalized Human Keratinocytes. Carcinogenesis. 2000; 21: 1087-1095.
- Gee K, Angel JB, Mishra S, Blahoianu MA, Kumar A. IL-10 regulation by HIV-Tat in primary human monocytic cells: involvement of
calmodulin/calmodulin-dependent protein kinase-activated p38
MAPK and Sp-1 and CREB-1 transcription factors. J Immunol. 2007;
178: 798-807.
- Li J, Jiang S, Chen Y, Ma R, Chen J, et al. Benzene metabolite hydroquinone induces apoptosis of bone marrow mononuclear cells
through inhibition of beta-catenin signaling. Toxicology in vitro: an
international journal published in association with BIBRA. 2018;
46: 361-369.
- Lofroth G. Environmental tobacco smoke: overview of chemical
composition and genotoxic components. Mutat Res. 1989; 222:
73-80.
- Fabiani R, De Bartolomeo A, Morozzi G. Involvement of oxygenfree radicals in the serummediated increase of benzo-quinone genotoxicity. Environ Mol Mutagen. 2005; 46: 156-163.
- Krewski D, Snyder R, Beatty P, Granville G, Meek B, et al. Assessing
the health risks of benzene: a report on the benzene state-of-the-science workshop. Journal of Toxicology and Environmental Health
Part A. 2009; 61: 307-308.
- Sims R, Vandergon VO, Malone CS. The mouse B cell-specific mb-1
gene encodes an immunoreceptor tyrosine-based activation motif (ITAM) protein that may be evolutionarily conserved in diverse
species by purifying selection. Mol Biol Rep. 2012; 39: 3185-3196.
- Kozlov I, Beason K, Yu C. CD79a expression in acute myeloid leukemia t(8;21) and the importance of cytogenetics in the diagnosis of
leukemias with immunophenotypic ambiguity. Cancer Genet Cytogenet. 2005; 163: 62-67.