Introduction
Digestive carcinoma chemotherapy regimens are mostly based
on fluoropyrimidine drugs (5-fluorouracil [5-FU] or capecitabine)
[1-5]. However, 5-FU is mainly catabolised by dihydro pyrimidine
dehydrogenase (DPD) [6], and partial or complete DPD activity
deficiency can cause severe adverse reactions including death [7].
Different strategies have been proposed to predict DPD
activity deficiency; the two main approaches are phenotyping
the enzyme activity (directly or indirectly), or genotyping the
four main polymorphisms of DPYD gene associated with 5-FU
toxicity [8-12]. In February 2018, the French medicines agency
(Agence nationale de sécuritédu médicament et des produits de
santé) recommended DPYD genotyping for all patients receiving
a fluoropyrimidine-based treatment to improve its safety as
did later the European Medicines Agency (EMA) [13] and other
pharmacogenetics working group. In contrast, the US Food and
Drug Administration chose not to require any regulatory review of
laboratory or genetic tests for use of 5-FU [14]. In December 2018
a new guideline from the French cancer institute (Institut National
Du Cancer, InCA) and the French health authority (Haute Autorité
de Santé, HAS) recommended the measurement of the plasma
uracil concentration, and, based on a consensus, dose adaptation
is required if this uracil level is between 16 and 150 ng/mL while
another drug should be considered if it is greater than 150 ng/
mL [15]. The aim was that phenotyping DPD activity could avoid
severe adverse reactions due to unknown DPYD variants that
impair DPD activity [16,17].
To our knowledge, no evaluation of this guideline in real-life
practice has been reported, which is of importance since 5-FU
displays a dose-response relationship regarding both its efficacy
and its toxicity [18,19]. To address that matter, we conducted a
retrospective study to evaluate how fluoropyrimidine dosage
was adapted to uracil concentration and its impact on patient
outcomes.
Materials and methods
Patients and study design
Patients were included in this multicentre retrospective study
if they had digestive cancer and an plasma uracil quantification
performed between February 2018 and January 2020, and
if they received at least one cycle of fluoropyrimidine-based
chemotherapy in one of the four participating oncology
departments (Hôpital Edouard Heriot [Lyon], Centre Hospitalier
de Lyon Sud [Lyon], Hôpital de la Croix Rousse [Lyon], Hôpital
Nord-Ouest de Villefranche-sur-Saone [Gleize]). The objective was to compare time to failure (TTF) and Overall Survival (OS)
among those with uracil <16 ng/ml to those with uracil ≥16
ng/ml. The following characteristics were collected from the
patient medical files: histology, stage (localised vs metastatic
disease), chemotherapy regimen, proportion of fluoropyrimidine
dose administered, fluoropyrimidine induced-toxicity, date of
progression and that of death (or last follow-up). The proportion
of fluoropyrimidine dose administered and adverse reactions of
fluoropyrimidine were assessed at cycle 1, 2 and 4 for those with
uracil ³16 ng/mL to characterise early (1st and 2nd cycles) and longterm (4th cycle) dose adaptation. Last active search for vital status
was March 30th 2021.
This is a non-interventional study and conducted according to
the guidelines of the Declaration of Helsinki, and registered by
the national data protection committee (Commission nationale
de l’informatique et des libertés [CNIL] in March 2021, number
21_5368).
DPD phenotyping
Plasma uracil concentration was quantified by high performance
liquid chromatography (HPLC) coupled with high resolution mass
spectrometry detection [20]. The results were analysed by a
senior biologist and the results of plasma uracil concentration
were available to clinicians within 8 to 10 days from initial patient
blood sample before the administration of treatment.
Statistical analysis
Data were described using median [interquartile range, IQR]
and mean (standard deviation, SD) for continuous variables,
and frequencies (percentage) for categorical variables. TTF and
progression-free survival (PFS) was defined as the time from the
first treatment with 5-FU / capecitabine to death or morphological
progression according to RECIST criteria or clinical progression
requiring a new anti-tumour treatment, whichever occurred first.
PFS was used for metastatic disease only. OS was defined as the
time from the first treatment with 5-FU / capecitabine to death
or last follow-up. Patients without these events were censored
at the time of last follow-up. TTF, PFS (for metastatic disease) and
OS were estimated using the Kaplan-Meier method. Univariate
analyses were performed using the Log-rank test for each variable
of interest. Multivariate analyses using a Cox proportional
hazards regression model were performed to identify factors
independently associated with prognosis. All significant factors
from the univariate analysis (Log-rank p<0.10) were included in
the multivariate analyses; p<0.05 was considered statistically
significant. The results from the survival analyses are presented
with the effect estimates, hazard ratios (HR), and 95% confidence interval (CI). All statistical analyses were performed using IBM-
SPSS version 21.
Results
Patient characteristics
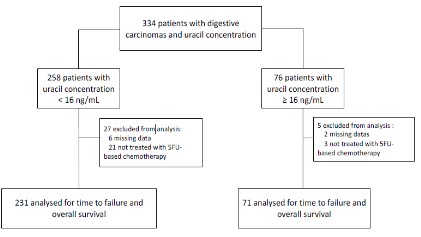
We identified a series of 334 patients with digestive cancers
with a known plasma uracil concentration; 32 were excluded for
missing data or lack of treatment with fluoropyrimidine (Figure 1).
Patients with a plasma uracil concentration ≥16 ng/mL
represented 23.5 % (71/302) of the total population. The two most
frequent digestive cancers were colorectal adenocarcinoma and
pancreatic adenocarcinoma; there was no significant difference
between groups except for the prevalence of squamous cell
carcinoma of oesophagus/anus (Table 1).
Fluoropyrimidine dose management of and toxicity evaluation
Among those with plasma uracil ³16 ng/mL, at cycle 1
continuous 5-FU or capecitabine dose was 0-50% of the theoretical
dose in 60.5% of patients, 51-75% in 15.5%, and 76-100% in 24%;
FU bolus was administered to 13.2% (9/68) of patients. Grade 3
or 4 fluoropyrimidine toxicity was observed in 2.8% of patients
(2/71) after cycle 1 (Table 2). Fluoropyrimidine increased dose
after a well-tolerated first cycle was observed for 7/69 (10.1%)
patients at cycle 2 and for 13/69 (18.8%) patients at cycle 4. Among
patients with plasma uracill <16 ng/mL, at cycle 1 the full dose of
continuous 5-FU or capecitabine was administered to 97.4% of
patients and 98.1% of patients received a 5-FU bolus. Grade 3 or 4
toxicity was experienced by 17 (7.4%) patients (Table 2).
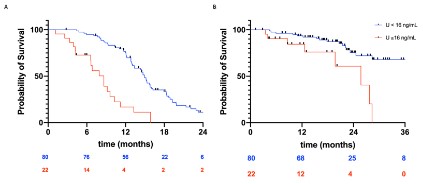
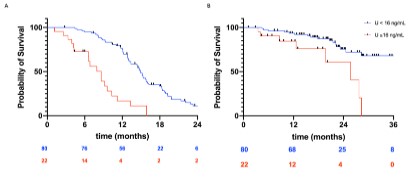
Survival analysis
The median TTF was estimated to be 8.6 months among those
with plasma uracil ³16 ng/mL, and 15.8 months among those with
uracil <16 ng/mL; the risk of tumour progression was significantly
higher among those with plasma uracil ³16 ng/mL (HR 0.38, 95%CI
[0.27; 0.53], p< 0.0001). The median OS was estimated to be 24.3
months among those with plasma uracil ³16 ng/mL, and 39.9
months among those with uracil <16 ng/mL; the risk of death was
significantly higher among those with uracil ³16 ng/mL (HR 0.46,
95%CI [0.29; 0.74], p=0.001; Figure 2).
Table 1: Patient characteristics according to plasma uracil concentration.
|
Uracil >_16 ng/mL |
Uracil <16 ng/mL |
P value |
| Number of patients |
71 |
231 |
|
| Median age (range) |
70.3 (49.7-86.2) |
67.5 (28-91) |
|
| Male |
44 (62%) |
144 (62.3%) |
0.96 |
| Cancer type |
| Colorectal ADK |
29 (40.9%) |
119 (51.5%) |
0.11 |
| Pancreatic ADK |
20 (28.1%) |
46 (19.9%) |
0.14 |
| SCC of oesophagus / anus |
11 (15.5%) |
10 (4.3%) |
0.001 |
| Gastric ADK |
4 (5.6%) |
31 (13.4%) |
0.07 |
| Neuroendocrine tumour |
4 (5.6%) |
15 (6.5%) |
0.79 |
| Other |
3 (4.2%) |
10 (4.3%) |
0.97 |
| Cancer stage |
| Localised |
25 (34.7%) |
81 (35.1%) |
0.96 |
| Metastatic |
47 (65.3%) |
150 (64.9%) |
0.96 |
ADK: Adenocarcinoma; SCC: Squamous cell carcinoma.
Table 2: Proportion of fluoropyrimidine dose administered and adverse reactions of fluoropyrimidine based on plasma uracil
concentration.
|
Uracil <16 ng/mL |
Uracil >16 ng/mL |
|
Cycle 1 (n=7) |
Cycle 2 (n=67) |
Cycle4 (n= 67) |
Cycle 1 (n=231) |
| % of continuous 5-FU or Capecitabine, n(%) |
| 0-50% |
43 (60.5) |
36 (53.7) |
36 (53.7) |
2 (0.9) |
| 51-75% |
11 (15.5) |
14 (20.4) |
17 (25.4) |
4 (1.7) |
| 76-100% |
17 (24) |
17 (25.4) |
14 (20.9) |
225 (97.4) |
| Patients with a bolus of 5FU |
9/68 (13.1) |
9 (13.1) |
7 (10.1) |
207/211 (98.1) |
| G3 or G4 loxicity |
2 (2.8) |
0 |
0 |
17(7.4) |
| Patients with an increased dose after well-tolerated first cycle |
- |
7/69(10.1) |
13/69 (18.8) |
- |
For patients with metastatic colorectal adenocarcinoma, the
median PFS and median OS were estimated to be, respectively
8.6 and 25.7 months among those with uracil ³16 ng/mL and 14.9
and 40.1 months among those with uracil <16 ng/mL; the risk
of progression and that of death was significantly higher among
those with uracil ³16 ng/mL (PFS: HR 0.26, 95%CI [0.11; 0.59], p
<0.0001; OS: HR 0.29, 95%CI 0.09-0.97, p=0.02, 174 Figure 3).
In multivariate analysis, the factors significantly associated
with TTF and OS were colorectal adenocarcinoma vs non-colorectal adenocarcinoma (HR for TTF 0.64, 95%CI [0.48; 0.86], p<0.003
and HR for OS 0.34, 95%CI [0.21; 0.54], p<0.0001), localised vs
metastatic cancer (HR for TTF 0.25, 95%CI [0.17; 0.36], p<0.0001
and HR for OS 0.26, 95%IC A B[0.14; 0.48], p<0.0001 ) and full
administrated fluoropyrimidine dose vs tailored (HR for TTF 0.14,
95%CI 0.06-0.34 and HR for OS 0.24, (95%IC [0.11; 0.55], p=0.001;
Tables 3 & 4).
Table 3: Factors associated with time to failure (TTF).
|
Univariate analysis |
Multivariate analysis |
|
Hazard ratio |
[95% CI] |
P value |
Hazard ratio |
[95% CI] |
P value |
| Age, < vs ³ median |
0.8 |
[0.60; 1.06] |
0.13 |
|
|
|
| Sex, female vs male |
0.92 |
[0.69; 1.23] |
0.59 |
|
|
|
| colorectal adenocarcinoma vs |
0.72 |
[0.54; 0.96] |
0.03 |
0.64 |
[0.48; 0.86] |
0.003 |
| Non-colorectal adenocarcinoma |
| Localised vs metastatic cancer |
0.26 |
[0.18; 0.38] |
<0.0001 |
0.25 |
[0.17; 0.36] |
<0.0001 |
| Fluoropyrimidine dose (full dose vs tailored*) |
0.23 |
[0.17; 0.33] |
<0.0001 |
0.14 |
[0.06; 0.34] |
<0.0001 |
| Plasma uracil concentration (<16 vs ³ 16) |
0.38 |
[0.27; 0.53] |
<0.0001 |
1.79 |
[0.74; 4.29] |
0.193 |
Table 4: Factors associated with overall survival (OS).
|
Univariate analysis |
Multivariate analysis |
|
Hazard ratio |
[95% CI] |
P value |
Hazard ratio |
[95% CI] |
P value |
| Age, < vs ³ median |
0.63 |
[0.41; 0.96] |
0.03 |
0.53 |
[0.34; 0.82] |
0.004 |
| Sex, female vs male |
0.88 |
[0.57; 1.38] |
0.59 |
|
|
|
| Colorectal adenocarcinoma vs non-colorectal adenocarcinoma |
0.38 |
[0.25; 0.60] |
<0.0001 |
0.34 |
[0.21; 0.54] |
<0.0001 |
| Localised vs metastatic cancer |
0.29 |
[0.16; 0.54] |
<0.0001 |
0.26 |
[0.14; 0.48] |
<0.0001 |
| Fluoropyrimidine dose (full dose vs tailored* ) |
0.28 |
[0.18; 0.45] |
<0.0001 |
0.24 |
[0.11; 0.55] |
0.001 |
| Plasma uracil concentration (<16 vs ³ 16) |
0.46 |
[0.29; 0.74] |
0.001 |
1.66 |
[0.73; 3.76] |
0.227 |
Discussion
In this retrospective cohort, we found that a tailored fluoropyrimidine dose impaired OS in patients with uracil ³16 ng/mL in
routine practice. Univariate analysis found that those with uracil
³16 ng/mL and those with decreased fluoropyrimidine dose had
a worse survival. As in multivariate analysis plasma uracil concentration was not associated to a worse prognosis, impaired survival in the plasma uracil ³16 ng/mL group was due to decreased
chemotherapy dosage in this population. By applying the French
recommendations in patients with uracil ³16 ng/mL, only 2.8 % of
patients herein experienced G3 or G4 toxicity at cycle 1 but from
baseline treatment dose was increased for only 18.8% of patients
at cycle 4. The present study highlights that we should consider
to increase more frequently the fluoropyrimidine dose administered after a well-tolerated first cycle. The results of the study
also emphasise that plasma uracil is not a prognostic factor but
that chemotherapy treatment displays a dose-effectiveness relationship as described in the literature [18]. However, many severe
toxicities induced by fluoropyrimidine can be explained by partial
DPD deficiency and complete DPD deficiency can lead to death
[8,11,21]. To improve the identification of patients at high risk of
toxicity, a combined composite biomarker should be proposed
based on both phenotyping and genotyping of the DPYD gene.
The latter is another way to evaluate DPD activity [22] and the
reported experience of systematic genotyping DPYD gene in reallife practice indicated that the administration of 5-FU at reduced
dose in patients heterozygous for DPYD*2A is safe [23]. The limitation of this technique is that in current clinical practice only four
variants are tested for 5-FU toxicity (DPYD*2A, DPYD*13, D949V
and HapB3), the use of which has failed to predict all cases of DPD
deficiency – possibly because other genes are implicated in 5-FU
toxicity and efficacy such as MTHFR, ABCB1 or TYMS [24,25,26].
Furthermore, to our knowledge, the impact of such testing in routine practice on survival has yet to be reported; only the impact
on toxicity has been published [26,27]. The main limitation of the
present study is its retrospective design that is associated with a
risk of confusion bias. In addition, the participating centres are
located in one administrative area of France, which may limit the
generalizability of the results. In the future, a model that associates phenotyping DPD and genotyping DPYD with other genes
of interest may be useful to better predict fluoropyrimidine toxicity and also to better adapt chemotherapy dosage.
Conclusions
The present study highlights that tailored fluoropyrimidine
dose impaired survival in patients with uracil 16 ng/mL and we
should consider to increase more frequently the fluoropyrimidine
dose administered after a well-tolerated first cycle. These results
should be confirmed by evaluating the clinical practice in the
whole French territory.
Declarations
Funding: This research received no external funding.
Institutional review board statement: This is a non-interventional study and conducted according to the guidelines of the Declaration of Helsinki, and registered by the CNIL in March 2021,
number 21_5368.
Informed consent statement: Informed consent was obtained
from all subjects involved in the study.
Data availability statement: To protect patient confidentiality,
the data will be consider for sharing only on written requests and
on case-by-case basis.
Acknowledgments: We thank Philip Robinson (DRS, Hospices
Civils de Lyon, Lyon, France) for help in manuscript preparation.
Conflicts of interest: The authors declare no conflict of interest.
Highlights
- Patients with uracil 16 ng/mL are treated with reduced
doses of fluoropyrimidine
- Fluoropyrimidine dose is not always increased after a well-tolerated first cycle
- Patients with a reduced fluoropyrimidine dose have worse survival
References
- André T, Meyerhardt J, Iveson T, Sobrero A, Yoshino T, et al. Effect
of Duration of Adjuvant Chemotherapy for Patients with Stage III
Colon Cancer (IDEA Collaboration): Final Results from a Prospective, Pooled Analysis of Six Randomised, Phase 3 Trials. Lancet Oncol. 2020; 21: 1620-1629.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, et al. FOL-
FIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N
Engl J Med. 2011; 364: 1817-1825.
- Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, et al. FOLFOXIRI plus Bevacizumab versus FOLFIRI plus Bevacizumab as
First-Line Treatment of Patients with Metastatic Colorectal Cancer:
Updated Overall Survival and Molecular Subgroup Analyses of the
Open-Label, Phase 3 TRIBE Study. Lancet Oncol. 2015; 16: 1306-1315.
- Lemelin A, Barritault M, Hervieu V, Payen L, Péron J, et al. O6-Methylguanine-DNA Methyltransferase (MGMT) Status in Neuroendocrine Tumors: A Randomized Phase II Study (MGMT-NET).
Digestive and Liver Disease. 2019; 51: 595-599.
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus
Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric
or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet. 2019; 393: 1948-1957.
- Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical
Pharmacokinetics of 5-Fluorouracil and Its Metabolites in Plasma,
Urine, and Bile. Cancer Res. 1987; 47: 2203-2206.
- Madi A, Fisher D, Maughan TS, Colley JP, Meade AM, et al. Pharmacogenetic Analyses of 2183 Patients with Advanced Colorectal
Cancer; Potential Role for Common Dihydropyrimidine Dehydrogenase Variants in Toxicity to Chemotherapy. Eur J Cancer. 2018;
102: 31-39.
- Fleming RA, Milano G, Thyss A, Demani F, Renà N. Correlation
between Dihydropyrimidine Dehydrogenase Activity in Peripheral
Mononuclear Cells and Systemic Clearance of Fluorouracil in Cancer Patients. Cancer Res. 1992; 52: 2899-2902.
- Gamelin E, Boisdron-Celle M, Guérin-Meyer V, Delva R, Lortholary A, et al. Correlation Between Uracil and Dihydrouracil Plasma
Ratio, Fluorouracil (5-FU) Pharmacokinetic Parameters, and Tolerance in Patients With Advanced Colorectal Cancer: A Potential
Interest for Predicting 5-FU Toxicity and Determining Optimal 5-FU
Dosage. Journal of Clinical Oncology. 1999; 17: 1105.
- Lu Z, Zhang R, Diasio RB. Dihydropyrimidine Dehydrogenase Activity in Human Peripheral Blood Mononuclear Cells and Liver: Population Characteristics, Newly Identified Deficient Patients, and
Clinical Implication in 5-Fluorouracil Chemotherapy. Cancer Res.
1993; 53: 5433-5438.
- Amstutz U, Farese S, Aebi S, Largiadèr CR. Dihydropyrimidine
Dehydrogenase Gene Variation and Severe 5-Fluorouracil Toxicity:
A Haplotype Assessment. Pharmacogenomics. 2009; 10: 931-944.
- Meulendijks D, Cats A, Beijnen JH, Schellens JHM. Improving Safety
of Fluoropyrimidine Chemotherapy by Individualizing Treatment
Based on Dihydropyrimidine Dehydrogenase Activity – Ready for
Clinical Practice? Cancer Treat Rev. 2016; 50: 23-34.
- Fluorouracil-Fluorouracil-Related-Substances-Article-31-Referral-Assessment-Report_en.Pdf.
- Health, C. for D. and R. Table of Pharmacogenetic Associations.
FDA 2020.
- Recherche de Déficit En Dihydropyrimidine Déshydrogenase EnVue de Prévenir Certaines Toxicités Sévères Survenant Sous Traitement Comportant Des Fluoropyrimidines (5-Fluorouracile), Recommandations et Référentiels, INCa, HAS, 2018.
- Cosmic DPYD Gene – COSMIC.
- Etienne-Grimaldi MC, Boyer JC, Beroud C, Mbatchi L, van Kuilenburg A, et al. New Advances in DPYD Genotype and Risk of Severe Toxicity under Capecitabine. PLoS One. 2017; 12.
- Kuilenburg ABPV, Lenthe H. van, Blom MJ, Mul EPJ, Gennip AHV.
Profound Variation in Dihydropyrimidine Dehydrogenase Activity
in Human Blood Cells: Major Implications for the Detection of
Partly Deficient Patients. Br J Cancer. 1999; 79: 620-626.
- Hodroj K, Barthelemy D, Lega JC, Grenet G, Gagnieu MC, et al. Issues and Limitations of Available Biomarkers for Fluoropyrimidine-Based Chemotherapy Toxicity, a Narrative Review of the Literature.
ESMO Open. 2021; 6: 100125.
- Tafzi N, Woillard JB, Fleytoux A, Picard N, Marquet P. Phenotyping
of Uracil and 5-Fluorouracil Metabolism Using LC-MS/MS for Prevention of Toxicity and Dose Adjustment of Fluoropyrimidines.
Ther Drug Monit. 2020; 42: 540-547.
- Kuilenburg ABP, van Haasjes J, Richel DJ, Zoetekouw L, Lenthe HV,
Abreu, R.A.D.; Maring, J.G.; Vreken, P.; Gennip, A.H. van Clinical
Implications of Dihydropyrimidine Dehydrogenase (DPD) Deficiency in Patients with Severe 5-Fluorouracil-Associated Toxicity:
Identification of New Mutations in the DPD Gene. Clin Cancer Res.
2000; 6: 4705-4712.
- Wei X, Elizondo G, Sapone A, McLeod HL, Raunio H, et al. Characterization of the Human Dihydropyrimidine Dehydrogenase Gene.
Genomics. 1998; 51: 391-400.
- Jolivet C, Nassabein R, Soulières D, Weng X, Amireault C, et al. Implementing DPYD*2A Genotyping in Clinical Practice: The Quebec,
Canada, Experience. Oncologist. 2021; 26: e597-e602.
- Lecomte T, Ferraz JM, Zinzindohoué F, Loriot MA, Tregouet DA, et
al. Thymidylate Synthase Gene Polymorphism Predicts Toxicity in
Colorectal Cancer Patients Receiving 5-Fluorouracil-Based Chemotherapy. Clin Cancer Res. 2004; 10: 5880-5888.
- Gonzalez-Haba E, García MI, Cortejoso L, López-Lillo C, Barrueco
N, et al. ABCB1 Gene Polymorphisms Are Associated with Adverse
Reactions in Fluoropyrimidine-Treated Colorectal Cancer Patients.
Pharmacogenomics. 2010; 11: 1715-1723.
- Nahid NA, Apu MNH, Islam R, Shabnaz S, Chowdhury SM, et al.
DPYD*2A and MTHFR C677T Predict Toxicity and Efficacy, Respectively, in Patients on Chemotherapy with 5-Fluorouracil for Colorectal Cancer. Cancer Chemother Pharmacol. 2018; 81: 119-129.
- Hishinuma E, Narita Y, Saito S, Maekawa M, Akai F, et al. Functional
Characterization of 21 Allelic Variants of Dihydropyrimidine Dehy-drogenase Identified in 1070 Japanese Individuals. Drug Metab.Dispos. 2018; 46: 1083-1090.
- Amstutz U, Henricks LM, Offer SM, Barbarino J, Schellens JHM, et
al. Clinical Pharmacogenetics Implementation Consortium (CPIC)
Guideline for Dihydropyrimidine Dehydrogenase Genotype and
Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther. 2018;
103: 210-216.