Introduction
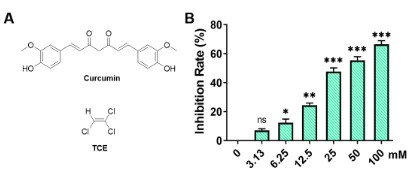
Trichloroethylene (TCE, Figure 1A) is a mass-produced
chemical that is widely used in many industrial applications,
such as in degreasing and dry cleaning of metal parts worldwide
[1,2]. Although its widespread use, this chemical is a ubiquitous
environmental pollutant [3]. TCE was classified in the list of
toxic and hazardous water pollutants in the year 2019. Despite
the recent regulations limit the use of TCE, its use has not been
completely banned [4]. The risk of TCE on human health aroused
widespread concern and its toxicity has been recognized and well-documented [5]. TCE exposure can lead to numerous types of
diseases, such as renal tubular injury, renal tubular epithelial cell
necrosis, liver damage, especially respiratory disease (asthma,
chronic bronchitis, rhinitis) [6-8].
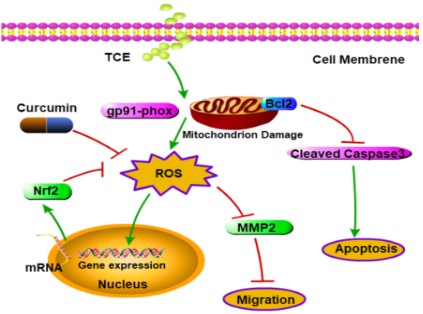
The toxicity of TCE has been considered to be caused by
generating excessive Reactive Oxygen Species (ROS) in the cellular
systems [9]. Under normal physiological conditions, oxidation
and reduction in the cells are in a stable state. When stimulated
by external toxic and harmful substances, free radicals will be
generated and the antioxidant system of cells will be activated.
When excess free radicals exceed the scavenging capacity of
cells, the homeostasis of cellular redox is unbalanced, resulting in
oxidative stress and oxidative damage to cells. Studies have shown
that oxidative stress is the mechanism of action for TCE-induced
liver damage [10,11]. TCE induces the generation of oxygen free
radicals through the metabolites of the CYP450 oxidation pathway,
which affects the activity of the body’s antioxidant enzymes, and
ultimately causes the body’s antioxidant system to be disordered
[9,12].
Curcumin (Cur, Figure 1A) is a polyphenolic compound extracted from the edible spice turmeric with various pharmacological effects [13]. It plays an important role in Indian and Chinese
traditional medicine for thousands of years [14]. In addition, experiments have shown that curcumin is safe in humans even at
high doses (12 g/day) [13]. In recent years, curcumin has received
more and more attention from scientists due to its antioxidant,
anti-inflammatory and anti-tumor properties [15]. Studies have
demonstrated promising therapeutic outcomes in cancer, cardiovascular disease, and immune disease including arthritis, uveitis,
ulcerative proctitis, and tropical pancreatitis [16]. And there is
evidence that cellular superoxide dismutase (SOD) activity is enhanced by curcumin treatment and can eliminate intracellular superoxide anion, reduce the level of ROS in cells, and reduce the
damage of oxidative stress to cells [17].
However, the protective effect and potential mechanisms of
curcumin after TCE exposure have not been fully investigated.
Therefore, we speculate that curcumin might have a protective
effect against TCE-induced A549 cell cytotoxicity. Cytotoxicity
assessments of TCE exposure (eg: cell viability, cell migration, ROS
characterization, mitochondrial membrane potential, apoptosis
levels) were performed on A549 cells pretreated or untreated
with curcumin.
Results
Effects of trichloroethylene on the viability of A549 cells
To assess the effect of TCE on the viability of A549 cells, A549
cells were treated with TCE at different concentrations of 0,
3.125, 6.25, 12.5, 25, 50, 100 mM for 24 h using an MTT assay.
The obtained results are shown in Figure 1B. TCE decreased the
viability of A549 cells in a dose-dependent manner. When the
concentration of TCE was 3.125 mM, there was no obvious toxic
effect of TCE on A549 cells. The viability of A549 cells treated with
25 mM and 50 mM TCE was significantly lower than that of control
(Ctrl) A549 cells. The results of cell viability assay showed that TCE
could inhibit the cell viability of A549 cells. The IC50 of TCE on A549
cells was calculated to be 21.74 mM. In further experiments, a TCE
concentration of 5 mM was chosen as the lowest concentration
for A549 cells. For curcumin, it has been experimentally proved
that the IC50 value of curcumin on A549 cells is around 20 μM, and
the final selected concentration is 20 μM [18].
Curcumin alleviates the inhibitory effect of TCE on cell
proliferation and migration
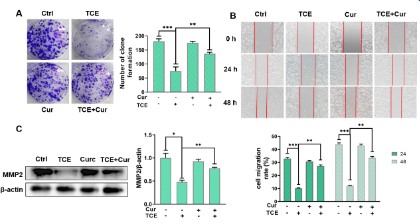
To investigate the effect of curcumin (20 μM) and/or TCE (5
mM) on the proliferation of A549 cells, a clone formation assay
was performed. After two weeks, the number of clones formed in
the control and curcumin-only groups was 181±6.9 and 176±4.1,
respectively (Figure 2A). While the TCE group was 75 ± 11.5, the
curcumin + TCE group was 132 ± 1.0. The results indicated that TCE
could significantly inhibit the proliferation of A549 cells with an
inhibition rate of 58.56% and the inhibition rate of TCE+curcumin
group was 27.07%. Curcumin reduced TCE-induced A549 cell proliferation by 31.49%, indicating that curcumin could alleviate the
inhibitory effect of TCE on A549 cells (p<0.05).
To verify the effect of curcumin (20 μM) and/or TCE (5 mM)
on the migration ability of A549 cells, a wound healing test was
carried out for 24 h and 48 h. The result is shown in Figure 2B.
The cell migration rates of the control group and curcumin-treated group were 32.93% and 30.67% at 24 hours, and 43.82% and
42.98% at 48 hours, respectively. However, the migration rates in
the TCE only treatment group at 24h and 48h were 10.15% and
12.12%, respectively. The migration rate of curcumin+TCE treatment group was 27.24% at 24h and 33.54% at 48h, which was
significantly higher than that of TCE only treatment group. The
results indicated that TCE exposure inhibited the migration ability
of A549 cells and curcumin could alleviate the inhibition of migration ability caused by TCE, which was in agreement with western
blot analysis (Figure 2C). The expression of MMP2 in A549 cells in
curcumin+TCE group was significantly higher than that in TCE-only
group. These results suggest that curcumin alleviates the inhibitory effect of TCE on A549 cell migration.
Curcumin inhibits intracellular ROS levels in A549 cells
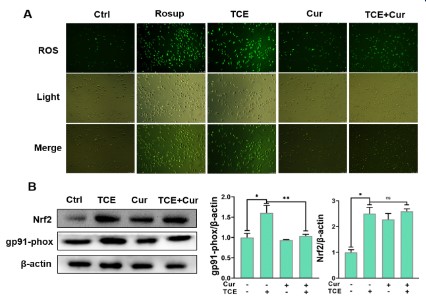
The change of intracellular ROS level was observed and analyzed by ROS assay. As shown in Figure 3A, A549 cells were loaded with ROS fluorescent probes and the green color represented
elevated intracellular ROS levels. Curcumin (20 μM)-treated cells
showed no significant change compared with the control group,
while TCE (5 mM) treated cells showed a significant increase in green fluorescence, which was very similar to the Rosup positive
control. However, the green fluorescence of curcumin+TCE group
was significantly decreased. The expressions of ROS-related proteins were demonstrated by western blot in Figure 3B. The expression of the oxidation marker gp91-phox in the curcumin+TCE
group was decreased compared with the TCE only exposure
group, while Nrf2 was significantly increased. Nrf2 is an essential
transcription factor that regulates an array of detoxifying and antioxidant defense gene expression in the liver. The results of ROS
assay and western blot indicated that the antioxidant system was
activated to inhibit the occurrence of ROS. In conclusion, curcumin can inhibit the increase of ROS level in A549 cells induced by
TCE (5 mM).
Curcumin protects membrane integrity and reduces apoptosis against TCE in A549 cells
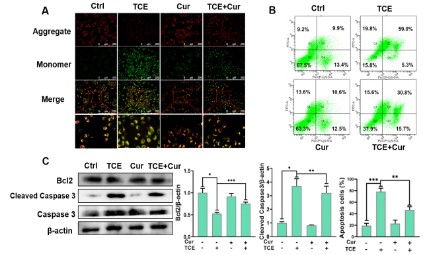
The degree of cell membrane damage was detected with the
mitochondrial membrane potential detection kit. In normal mitochondria, JC-1 forms aggregate in the mitochondrial matrix and
emits strong red fluorescence. In lesions, JC-1 can only exist in
the cytoplasm as a monomer, producing green fluorescence [19].
A549 cells treated with curcumin (20 μM) and/or TCE (5 mM)
were stained with JC-1 staining solution. The results are shown
in Figure 4A. Mitochondrial changes were not significantly different between the control group and the curcumin-only group. In
contrast, the green fluorescence in the TCE exposure group was
significantly increased, which suggests that the mitochondrial
membrane of the cell is damaged. The green fluorescence of cells
in the curcumin + TCE group was significantly reduced compared
with the TCE alone treatment group, which indicated that curcumin could alleviate the membrane potential damage caused by
TCE.
To investigate the effect on the apoptosis ability of A549 cells,
the apoptosis rate of A549 cells after TCE (5 mM) and/or curcumin
(20 μM) treatment for 24 h was determined using Annexin V-FITC/
PI staining (Figure 4B). The results showed that the apoptosis rates
of A549 cells in the control and curcumin-treated groups were 19.4 ± 3.23% and 23 ± 4.93%, respectively. The apoptosis rate
of A549 cells in TCE treatment group was 78.4 ± 4.78%, and the
apoptosis rate of cells treated with curcumin (20 μM) and TCE (5
mM) was significantly reduced by 46.6 ± 4.49%. These results suggest that curcumin can effectively protect A549 cells from TCE-induced apoptosis. The results of western bolt in Figure 4C showed
that the expression of the pro-apoptotic protein cleaved caspase
3 in the TCE treatment group was significantly increased, and
the expression of the anti-apoptotic protein Bcl2 was decreased.
However, the curcumin + TCE treatment group decreased the expression of cleaved caspase 3 and increased Bcl2, which indicated
that curcumin could induce apoptosis against TCE in A549 cells.
Discussion
Trichloroethylene (TCE) is an occupational high-risk poison and
many workers die from TCE occupational hazards every year due
to its widespread use [20]. The toxicity of TCE to cells is mainly
caused by intracellular oxidative stress, which in turn leads to oxidative damage to cells and eventually leads to cell death [8].
Curcumin, as a natural compound, has been confirmed to have
various pharmacological activities such as anti-inflammatory,
antioxidant, and anti-tumor [21,22]. This study mainly explores
that curcumin reduces TCE-induced cytotoxicity through its antioxidant capacity. Curcumin works by enhancing anti-oxidative
intracellular defense system, alleviating the inhibitory effect of
TCE on cell proliferation and migration, improving cell membrane
integrity and regulating apoptosis.
Our study showed that 5mM TCE decreased the viability of
A549 cells. In cell cloning experiments and scratch healing experiments, TCE exhibited inhibitory effects on the proliferation and
migration of A549 cells (Figures 2A, 2B). Compared with the TCE
group, the proliferation and migration ability of the cells in the
curcumin+TCE group were significantly restored. We also detected the cell migration-related protein MMP2 by western bolt.
MMP2 is involved in angiogenesis by disrupting basal layer molecules and remodeling the extracellular matrix during angiogenesis. Involved in cell migration activities, high expression of MMP2
makes it easier for tumor cells to invade surrounding tissues and
metastasize to secondary sites [23]. Therefore, the detection of
MMP2 protein allows us to effectively evaluate the migration ability of cells. In the TCE exposure group, the expression of MMP2
protein was significantly decreased (Figure 2C). Significant recovery of MMP was obtained in the curcumin+TCE group. That is, the
migratory ability of the cells is restored. The experimental results
indicated that curcumin could relieve the inhibition of A549 cell
proliferation and migration ability induced by TCE.
Many researchers have reported that the toxic effects of TCE
on various cells are mainly inducing oxidative stress and leading
to apoptosis [9]. When cells are exposed to toxic substances such
as TCE, the antioxidant system in the cell is activated and can produce antioxidants (such as enzymes, vitamins and flavonoids) to
neutralize intracellular superoxide anions and maintain a stable
intracellular environment. When the level of intracellular oxidation is too high, there will be an imbalance between oxidation and
anti-oxidation. As a result, the excess superoxide anion in the cell
cannot be removed, attacking the cell and causing damage to the
cell [24]. While curcumin benefits from its antioxidant pharmacological action, it can effectively remove excess free radicals in
cells, relieve intracellular oxidation levels and protect cells from
damage [21].
In this study, we measured the level of oxidation inside the
A549 cell. The reactive oxygen species detection kit is a commonly used reactive oxygen species detection method and intracellular oxidative stress can be detected by the kit in the form
of green fluorescence. The results of this study showed that the
fluorescence level of A549 cells exposed to TCE was significantly
increased under microscope, and close to the positive control
group (Figure 3A). The green fluorescence level of A549 cells in
the curcumin+TCE group was significantly lower than that in the
TCE-treated group, which indicated elevated levels of oxidative
stress in cells. In addition, gp91-phox protein, as a marker of oxidative stress, was detected by western bolt analysis (Figure 3B).
Compared with the increase in the expression of gp91-phox in the
TCE group, it could be proved that the co-exposure of curcumin
and TCE could significantly reduce the level of intracellular oxidative stress.
Recently, many scientific researchers reported that curcumin
exhibited strong protective effects through the Keap1/Nrf2 signaling pathway. Nrf2 is considered to be an emerging and important
regulator of cellular antioxidants [25]. Our results showed that
the expression of Nrf2 in cells was significantly increased in the
TCE exposure group, while the expression of Nrf2 in the curcumin
+ TCE treatment group was higher than that in the control group.
This means that the antioxidant system in the cell is activated and
plays an important role in scavenging peroxides in cells. Oxidative stress induced by TCE is alleviated by the antioxidant effect
of curcumin (Figure 3B). Our findings are similar to those reported by previous research [26]. They demonstrated that curcumin
protected PC12 cells from arsenic-induced oxidative damage by
activating Nrf2. Therefore, curcumin protected A549 cells from
TCE-induced oxidative stress.
TCE can cause cell death and the way is through damaging and
destroying the mitochondrial membrane of cells. The apoptosis
rate of A549 cells in the TCE treatment group was significantly
increased by JC-1 experiments and flow cytometry in Figure 4A,
4B. The apoptosis rate of A549 cells in the curcumin + TCE group
was significantly lower than that in the TCE group. We also investigated proteins associated with apoptosis in Figure 4C. Members
of the Bcl2 family play important regulatory roles in mitochondrial membrane permeability and can inhibit cell apoptosis [27].
Caspase 3 protein is a key protein in the apoptotic pathway, and
cleaved caspase 3 can be activated to induce cell apoptosis [28].
The result showed that TCE exposure group significantly increased
the expression of the pro-apoptotic protein cleaved caspase 3 and
decreased the expression of the anti-apoptotic protein Bcl2. Similar findings were also reported [26]. In the curcumin + TCE group,
the decreased expression of cleaved caspase 3 and the increase
of Bcl2 indicated that curcumin successfully reduced TCE-induced
apoptosis in A549 cells.
In conclusion, we believe that cell apoptosis induced by TCE is
closely related to oxidative stress. Curcumin works by scavenging
excess free radicals and reducing oxidative stress levels in cells.
Materials and methods
Chemicals and materials
Trichloroethylene (CAS No. 79-01-6) (Aladdin, Shanghai, China)
and curcumin (CAS No. 458-37-7) (Beijing Lujia Technology Co.,
Ltd, Beijing, China) were dissolved by dimethyl sulfoxide (DMSO)
(St. Louis, Mo, USA). The second antibody was purchased from
cell signaling technology (CST) (Danvers, Ma, USA). Antibodies to
MMP2 (ab92536), Nrf2 (ab62352), gp91-phox (ab129068), Bcl2
(ab182858), cleaved caspase 3 (ab32042) were purchased from
Abcam Biological Technology (USA). Antibodies to caspase 3
(#14220) were purchased from Cell Signaling Technology (USA).
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazole bromide (MTT)
was purchased from Sigma (USA).
Cell culture and treatment
The human lung cancer cell line A549 was purchased from
ATCC. The cell lines were cultured in DMEM medium (Biosharp,
Lanjieke, Beijing) supplemented with 10% fetal bovine serum (Sijiqing Bioengineering Materials, Hangzhou, China). The cells were
placed in a 37°C incubator with 5% of CO2.
Cell viability analysis
Cell viability assay of TCE to A549 cell was carried out using
96-well plate cultures and MTT staining [29]. Briefly, the A549 cell
line (10000 cells/well) was treated with TCE at different concentrations (0, 3.125, 6.25, 12.5, 25, 50, 100mM) in 96-well culture
plates for 24 h. Then 10 μL of MTT (5 mg/mL MTT in PBS) solution
was added to each well, and the microplates were further incubated at 37°C for 4 h. After carefully removing the medium, the precipitates were dissolved in 200 μL of DMSO in the dark, shaken mechanically for 10 min, and then absorbance values at a wavelength
of 490 nm were taken on a Super Microplate Reader (MQX2OO)
(BioTek, the United States). Cell inhibition rate (%) = 1- [(Experimental group - Blank group) / (Control group - Blank group)] ×
100%. IC50 values were calculated through nonlinear fitting in GraphPad Prism 8 software. The results were determined from replicates of 96 wells from at least three independent experiments.
Clone formation assay
Cell clone formation assay is an important technical method
used to detect cell proliferation ability [30]. A549 cells were seeded in 6-well plates (approximately 50 cells/well). They were
treated with curcumin and/or TCE and placed in a cell incubator.
When a single cell proliferates for more than 6 passages, it can
form a cell population that is visible to the naked eye. The former
colonies were fixed with 4% paraformaldehyde and stained with
0.1% crystal violet. The number of cell colonies was counted and
compared with the control group.
Wound-healing assay
A wound healing test was used to evaluate the effects of curcumin on tumor cell motility by TCE-induced A549 tumor cell lines
[31]. A549 cells (2 × 105 cells/well) were seeded in 6-well plates
containing 15% fetal bovine serum medium. Treated with curcumin (20 μM) and/or TCE (5 mM), cultured in a cell incubator at
37°C, 5% CO2. When the cells grow to about 80%, use a 200 μM
pipette tip to draw a straight line in the 6-well plate, and use PBS
to remove unattached or damaged cells. The width of the scratched area was then photographed with an optical microscope at
0h, 24h and 48h.
Intracellular ROS levels measurement
The ROS detection kit was used to analyze the changes of ROS
levels in A549 cells exposed to TCE. Briefly, A549 cells were loaded
with probes first and then treated with curcumin (20 μM) and/
or TCE (5 mM). Rosup was added to new A549 wells as a positive
control for 1.5 hours of dosing stimulation. After 2 hours, the cell
culture medium was changed to 1 × PBS, and the ROS level of
A549 cells was quantitatively observed under a fluorescence microscope (Leica, Wetzlar, Germany).
Mitochondrial membrane damage detection
Cells were seeded in 24-well plates at a cell density of 2 × 105
cells/well. The cells were pretreated with curcumin and/or TCE
and then stained with the pre-configured JC-1 dye pair. After
shaking gently, the 24-well plate was placed in an incubator for
20 minutes. The plate was then washed and 1 × PBS was added.
Mitochondrial membrane damage was observed under a fluorescence microscope.
Flow cytometry analysis
Annexin V-FITC Apoptosis Detection Kit (BioVision, USA) was
used. A549 cell death was analyzed using flow cytometry. Briefly, A549 cells treated with curcumin (20 μM) and/or TCE (5 mM)
were cultured for 24 h and washed with 1 × PBS. After washing,
cells were suspended in 500 μL of 1× binding buffer containing 5
μL Annexin V-FITC and propidium iodide (PI). The resulting solution was then inhibited for 5 minutes in the dark. Finally, A549
cell samples were analyzed by flow cytometry (BD FACSVerse, BD
Biosciences).
Western blot
Western blot analysis was performed according to the previous
reference [32]. Proteins from A549 cells that had been treated
with curcumin and/or TCE for 24 hours were extracted. They were
separated using 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to PVDF membranes. Incubate
with primary antibodies against MMP2, gp91-phox, caspase 3,
cleaved caspase 3, Bcl2, Nrf2 for 12 hours at 4°C. Membranes
were then washed three times with TBST and incubated with HP-conjugated goat anti-rabbit or anti-mouse IgG for 1.5 h at room
temperature. After washing with TBST to remove excess antibody,
the expressions of the target proteins were detected by a chemiluminescence detection system according to the instructions.
Statistical analysis
All experiments were performed three times independently
under the same conditions and were expressed as the mean standard error (SEM) of the mean. The T test (p=0.05) was used to determine the statistical significance between the two groups. SPSS
software was used for all statistical analyses. Differences were
considered statistically significant if the p-value was less than 0.05
(*p<0.05, **p<0.01, ***p<0.001).
Conclusion
In conclusion, curcumin may regulate the expression of apoptosis-related proteins and enhance the antioxidant defense system
to inhibit the generation and accumulation of ROS. Thus, A549
cells are effectively protected from TCE-induced oxidative damage
and cytotoxicity. It can be seen that the natural compound curcumin has strong antioxidant and anti-apoptotic effects. These
findings suggest that curcumin would be a potential and safe
therapeutic agent to combat TCE toxicity. Further studies on the
interaction and protective mechanisms of curcumin against TCE-induced toxicity are warranted.
Declarations
Conflicts of interest: The authors declare that they have no
conflicts of interest.
Funding: This work was supported by Anhui Provincial Natural Science Foundation (NO. 2108085QH381), Provincial Natural
Science Research Program of Higher Education Institutions of
Anhui Province (No. KJ2021A0436), Research Start-up Fund for
High-level Introduced Talents of Anhui University of Science and
Technology (NO. 13200422), Practice Innovation Training Program
for College Students in Anhui Province (NO. S202110361220).
References
- Dumas O, Despreaux T, Perros F, Lau E, Andujar P, et al. Respiratory
effects of trichloroethylene. Respir Med. 2018; 134: 47-53.
- Siggins A, Abram F, Healy MG. Pyrolysed waste materials show potential for remediation of trichloroethylene-contaminated water. J
Hazard Mater. 2020; 390.
- Jollow DJ, Bruckner JV, McMillan DC, Fisher JW, Hoel DG, et al. Trichloroethylene risk assessment: a review and commentary. Crit
Rev Toxicol. 2009; 39: 782-797
- Cichocki JA, Guyton KZ, Guha N, Chiu WA, Rusyn I, et al. Target
Organ Metabolism, Toxicity, and Mechanisms of Trichloroethylene
and Perchloroethylene: Key Similarities, Differences, and Data
Gaps. J Pharmacol Exp Ther. 2016; 359: 110-123.
- Ordaz JD, Damayanti NP, Irudayaraj JMK. Toxicological effects of
trichloroethylene exposure on immune disorders. Immunopharmacol Immunotoxicol. 2017; 39: 305-317.
- Wang Y, Huang M, Du X, Hong Y, Huang L, et al. Renal tubular cell
necroptosis: A novel mechanism of kidney damage in trichloroethylene hypersensitivity syndrome mice. J Immunotoxicol.
2021; 18: 173-182.
- Ren XH, Chen Z, Ruan J, Zhong J, Deng R, et al. Trichloroethylene-induced downregulation of miR-199b-5p contributes to SET-mediated apoptosis in hepatocytes. Cell Biology and Toxicology. 2019;
35: 565-572.
- Li B, Xie H, Wang X, Yang X, Yang L, et al. Oxidative stress mediates
renal endothelial cell damage in trichloroethylene-sensitized mice.
J Toxicol Sci. 2019; 44: 317-326.
- Lash LH, Chiu WA, Guyton KZ, Rusyn I. Trichloroethylene biotransformation and its role in mutagenicity, carcinogenicity and target
organ toxicity. Mutat Res Rev Mutat Res. 2014; 762: 22-36.
- Ren X, Yang X, Hong WX, Huang P, Wang Y, et al. Identification of
the proteins related to SET-mediated hepatic cytotoxicity of trichloroethylene by proteomic analysis. Toxicol Lett. 2014; 227: 12-19.
- Hu C, Jiang L, Geng C, Zhang X, Cao J, et al. Possible involvement
of oxidative stress in trichloroethylene-induced genotoxicity in human HepG2 cells. Mutat Res. 2008; 652: 88-94.
- Cummings BS, Zangar RC, Novak RF, Lash LH. Cytotoxicity of trichloroethylene and S-(1, 2-dichlorovinyl)-L-cysteine in primary
cultures of rat renal proximal tubular and distal tubular cells. Toxicology. 2000; 150: 83-98.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007; 4:
807-818.
- Fabianowska-Majewska K, Kaufman-Szymczyk A, Szymanska-Kolba
A, Jakubik J, Majewski G et al. Curcumin from Turmeric Rhizome:
A Potential Modulator of DNA Methylation Machinery in Breast
Cancer Inhibition. Nutrients. 2021; 13.
- Almatroodi SA, Syed MA, Rahmani AH. Potential Therapeutic Targets of Curcumin, Most Abundant Active Compound of Turmeric
Spice: Role in the Management of Various Types of Cancer. Recent
Pat Anticancer Drug Discov. 2021; 16: 3-29.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin:
lessons learned from clinical trials. AAPS J. 2013; 15: 195-218.
- Zia A, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S.
The role of curcumin in aging and senescence: Molecular mechanisms. Biomed Pharmacother. 2021; 134: 111119.
- Zhang W, Bai W, Zhang W. MiR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell
lung cancer A549 cells. Clin Transl Oncol. 2014; 16: 708-713.
- Han X, Peng B, Xiao BB, Cao SL, Yang CR et al. Synthesis and evaluation of chalcone analogues containing a 4-oxoquinazolin-2-yl
group as potential anti-tumor agents. Eur J Med Chem. 2019; 162: 586-601.
- Alexander DD, Kelsh MA, Mink PJ, Mandel JH, Basu R, et al. A meta-analysis of occupational trichloroethylene exposure and liver
cancer. Int Arch Occup Environ Health. 2007; 81: 127-143.
- Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Molecular Targets and Therapeutic Uses of
Curcumin in Health and Disease. 2007; 595: 105-125.
- Zhao S, Pi C, Ye Y, Zhao L, Wei Y. Recent advances of analogues
of curcumin for treatment of cancer. Eur J Med Chem. 2019; 180:
524-535.
- Ma Y, Xu R, Liu X, Zhang Y, Song L et al. LY3214996 relieves acquired
resistance to sorafenib in hepatocellular carcinoma cells. Int J Med
Sci. 2021; 18: 1456-1464.
- Khan S, Priyamvada S, Khan SA, Khan W, Farooq N, et al. Effect
of trichloroethylene (TCE) toxicity on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in
kidney and other rat tissues. Food Chem Toxicol. 2009; 47: 1562-1568.
- Serafini MM, Catanzaro M, Fagiani F, Simoni E, Caporaso R, et al.
Modulation of Keap1/Nrf2/ARE Signaling Pathway by Curcuma-and Garlic-Derived Hybrids. Front Pharmacol. 2019; 10: 1597.
- Rahaman MS, Banik S, Akter M, Rahman M, Sikder T, et al. Curcumin alleviates arsenic-induced toxicity in PC12 cells via modulating
autophagy/apoptosis. Ecotoxicol Environ Saf. 2020; 200: 110756.
- Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2
proteins and apoptosis: an update. Archives of Toxicology. 2015;
89: 289-317.
- Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011; 351:
41-58.
- Yu P, Li DD, Ni JJ, Xia CJ, Wang ZZ, et al. Synthesis and Antitumor Activity of C-3(R) Hydroxy Modified Betulinic Acid Derivatives. Chem
Nat Compd+. 2019; 55: 1080-1084.
- Cao W, Gao J, Zhang Y, Li A, Yu P, et al. Autophagy up-regulated by
MEK/ERK promotes the repair of DNA damage caused by aflatoxin
B1. Toxicol Mech Methods. 2021; 1-10.
- Tabolacci C, Lentini A, Mattioli P, Provenzano B, Oliverio S, et al.
Antitumor properties of aloe-emodin and induction of transglutaminase 2 activity in B16-F10 melanoma cells. Life Sci. 2010; 87:
316-324.
- Yu P, Xia CJ, Li DD, Ni JJ, Zhao LG, et al. Design, synthesis, and anti-inflammatory activity of caffeoyl salicylate analogs as NO production inhibitors. Fitoterapia. 2018; 129: 25-33.