Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in industrialized countries with incidence rates rising due
to aging and obesity. Most patients are diagnosed with low-grade
EC (grade 1-2 endometrioid EC), and generally have a favorable
prognosis [1]. Around 20% of patients are diagnosed with highgrade EC (grade 3 endometrioid EC and non-endometrioid EC),
have an overall poor prognosis and are associated with an increased risk of regional or distant metastases [1]. Currently, primary surgical treatment is based on preoperative tumor grade
and histology.
According to the recent ESGO/ESTRO/ESP (European Society
of Gynaecological Oncology – European SocieTy for Radiotherapy
and Oncology – European Society of Pathology) guideline, adjuvant treatment is based on risk classification groups incorporating FIGO (Federation International of Gynecology and Obstetrics)
stage, tumor grade and histology, Lymphovascular Space Invasion
(LVSI) and with or without molecular markers [2]. Often routinely
obtained preoperative clinical biomarkers, such as hematological
parameters, may contribute to identification of patients with extended disease and/or aggressive tumor behavior that might respond differently to adjuvant therapy [3-5].
Endometrial carcinogenesis is characterized by chronic inflammation with elevated pro-inflammatory cytokines and acute
phase proteins [6]. Overexpression of inflammatory cytokines
could contribute to the development of cancer-related anemia,
thrombocytosis and leukocytosis, and could generate a protumorigenic environment [7-10]. Preoperative abnormal hematological
parameters like anemia, thrombocytosis and/or leukocytosis, have
been shown to be associated with FIGO advanced-stage and unfavorable outcome, however results remain conflicting [8,9,11-16].
Several studies showed an adverse impact of anemia to Radio
Therapy (RT) response in solid tumors, explained by the fact that
anemia is proposed to be a surrogate maker for tumor hypoxia
[4,17]. Hypoxia is very common in solid tumors and leads to cellular stress response, which allows tumor cells to survive. In addition, these hypoxic conditions may also protect tumor cells from
downstream DNA breaks and lethality induced by radiotherapy
[18,19]. Within gynecological tumors, leukocytosis was also observed to have an adverse predictive impact on RT response [5].
So far, no studies reported the impact of thrombocytosis on RT in
solid tumors.
Based on conflicting results in outcome of abnormal preoperative hematological parameters in endometrial cancer, we aim to
evaluate the prognostic relevance of anemia, thrombocytosis and
leukocytosis on survival. Second, we aim to explore the predictive
relevance of these abnormal hematological parameters on response to adjuvant RT. We hypothesize that patients with anemia
and thrombocytosis have reduced survival due to advanced stage
and/or disseminated EC, and anemia might have negative impact
on response to adjuvant RT.
Material and methods
Study cohort
A multicenter cohort study was performed with a combination of prospective and retrospectively collected data in patients
diagnosed with EC. This study is a collaboration between the
Netherlands and the United Kingdom (UK) by which data of nine
hospitals in the Netherlands (PIpelle Prospective ENDO metrial
carcinoma (PIPENDO) cohort) [20] and one in the UK [21] were
merged. The design and patient cohort of both cohorts, including
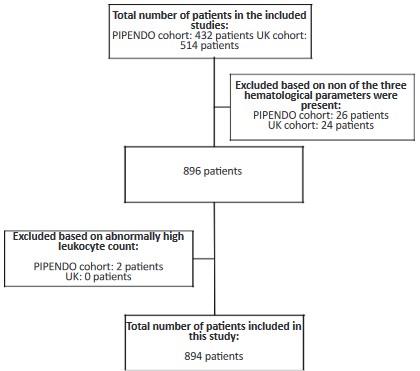
946 patients in total (PIPENDO and UK), have been published previously [20,21]. A study flowchart is shown in the Figure S1.
Data collection
All patients were surgically treated between 2006-2015. For
the Dutch participating hospitals patient characteristics, posto-perative tumor histology, grade and FIGO staging were collected
prospectively [20]. Preoperative hemoglobin level, platelet- and
leukocyte counts were collected retrospectively from hospital
records. For the UK center, all clinicopathological characteristics
and preoperative hematological parameters were collected retrospectively [21]. Regarding the data collection of nodal status,
in the Netherlands and UK surgical staging is selectively performed in patients with preoperative high-grade histology (grade 3
endometrioid EC and non-endometrioid EC) and in case of clinical
suspicion of extended disease, according to the Dutch and British
EC guideline [22,23].
The sole additional inclusion criteria used for this study was
that patients were only included if at least one of the three preo-perative hematological parameters was conducted ≤6 weeks prior
to surgery, resulting in 896 patients.
Statistical analysis
The hematological parameters were analyzed as a dichoto-
mous value, with defined cut-offs. Anemia was defined accor-
ding to the World Health Organization as hemoglobin level <7.45
mmol/L (<12 g/Dl) [24]. Thrombocytosis as platelet counts >400 x
109 /L according multiple studies involving gynecologic malignancies8 and leukocytosis as leukocyte counts >10 x 109/L [10].
The risk classification groups were classified according to the
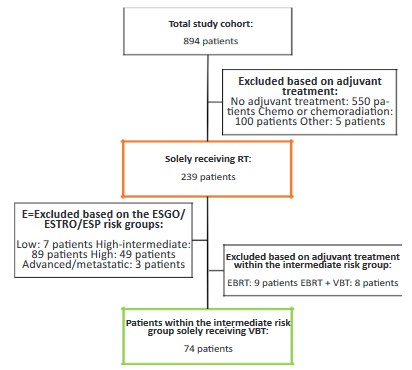
ESGO/ESTRO/ESP guideline; low, intermediate, high-intermediate, high and advanced/metastatic risk group [2]. To explore the
response on RT, all patients who received solely adjuvant RT were
included for the second analysis. To further refine response of RT
and in order to prevent treatment bias by including patients who
were not treated according to the recent guideline, patients only
classified as ESGO/ESTRO/ESP intermediate risk were included
(flowchart secondary analysis Figure S2). According to the guideline, these patients are recommended to receive adjuvant Vaginal
Brachytherapy (VBT) [2]. Whereas other risk classification groups
include observation or combined chemoradiotherapy.
For statistical analyses, Statistical Package for the Social
Sciences, version 25.0 (IBM, New York, NY, USA) was applied.
The results were considered significant with P-value less than
0.05 (P<0.05). Clinicopathological characteristics between dichotomous hematological subgroups were compared using the χ2
or Fisher’s exact test for categorical data, and the non-parametric Mann-Whitney U-test for continuous variables. Association
between exposure and outcome are shown as Odds Ratio (OR),
95% Confidence Interval (CI) and P-value. Survival analyses were
performed using Kaplan-Meier curves and univariable and multi-variable Cox-regression. Associations are shown as Hazard Ratio
(HR), 95% CI and P-value. Disease-Specific Survival (DSS) was defined as time from date of diagnosis to date of death by EC and
Recurrence-Free Survival (RFS) was defined as time from surgery
to time of recurrence from EC disease, all censored by date of last
contact.
Results
Patients
A total of 896 EC patients were included with a least one hematological parameter. Two patients had abnormally high leukocyte count (>50 x 109/L) due to chronic lymphatic leukemia and
unknown cause, these patients were excluded, resulting in 894
EC patients (54.8% British and 45.2% Dutch) included in this study with a median follow-up of 4.5 years (range 0-10 years) (Figure S1). Clinicopathological characteristics of the study cohort
are shown in Table 1. Median age was 65.9 (27.2-93.8) years and
median body mass index 29.7 (16.4-60.9) kg/m2. Of 653 (73.0%)
EC patients all three hematological parameters were available.
Median preoperative hemoglobin level was 8.4 mmol/L, median
platelet count 298.3 x 109 platelets/L and median leukocyte count
8.1 x 109/L. Anemia was present in 103 (11.3%), thrombocytosis in
79 (8.6%) and leukocytosis in 114 patients (12.5%). Most patients
were diagnosed with low-grade (grade 1-2), FIGO stage I-II and
endometrioid EC (respectively, 69.4%, 90.2% and 82.2%). Lymphadenectomy was performed in 205 patients (22.9%) of whom
34 (16.5%) had lymph node metastasis. Adjuvant treatment was
administered in 344 patients (38.5%). A total of 239 patients
(69.5%) received RT of which 132 patients (55.2%) VBT and 107
patients (44.8%) external beam radiation therapy with or without
VBT. Hundred and twenty-four patients (13.9%) developed recurrent EC, and 160 patients (17.9%) were deceased of which 99
(61.8%) deaths were directly related to EC.
Preoperative hemoglobin-, platelet- and leukocyte level in relation to clinicopathological characteristics are shown in Table 2.
Hemoglobin level was measured in 894 (100.0%), platelet count
in 721 (80.6%) and leukocyte count in 667 patients (74.6%). Patients with anemia were significantly associated with grade 3 EC
(OR 1.81, 95% CI 1.18-2.79), LVSI (OR 1.61, 95% CI 1.00-2.57), and
ESGO/ESTRO/ESP high risk (OR 2.11, 95% CI 1.30-3.42). The presence of thrombocytosis was significantly associated with LVSI
(OR 1.77, 95% CI 1.04-2.99), and ESGO/ESTRO/ESP high risk (OR
1.78, 95% CI 1.02-3.11). Leukocytosis was significantly associated
with ESGO/ESTRO/ESP advanced/metastatic risk (OR 2.72, 95% CI
1.06-6.97).
Outcome
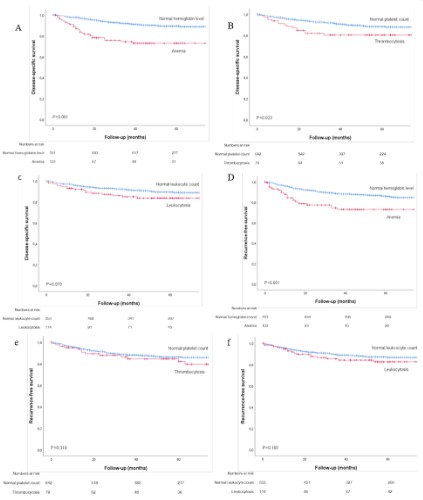
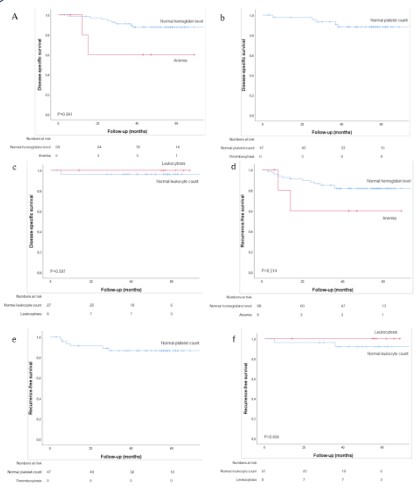
The 5-year DSS and RFS of preoperative anemia, thrombocytosis and leukocytosis are shown in Figure 1A-F. Patients with anemia had a significant reduced 5-year DSS and RFS compared to
patients with normal hemoglobin level (respectively, P<0.001 and
P<0.001) (Figure 1A,1D). Patients with thrombocytosis showed significant reduced 5-year DSS compared to normal platelet count
(P=0.023), no difference was found for RFS (Figure 1B,1E). For patients with leukocytosis compared with normal leukocyte count,
no significant difference in DSS and RFS was found (Figure 1C,1F).
In multivariable analysis after adjusting for age, the three abnormal hematological parameters and the ESGO/ESTRO/ESP risk
groups, only anemia, age and ESGO/ESTRO/ESP high- and advanced/metastatic risk groups remained independently associated with a reduced DSS. None of the hematological parameters
were independently associated with a decreased RFS (Table 3).
Table 1: Baseline clinic pathological characteristics.
| Patient characteristics |
|
Total (n=894) |
| Age (years) |
|
65.9 (27.2-93.8) |
| BMI (kg/m2) |
|
29.7 (16.4-60.9) |
| Serum values |
|
|
| Hemoglobin mmol/L |
|
8.4 (3.9-10.6) |
| Hemoglobin <7.45 mmol/L |
|
103 (11.3) |
| Platelets x 109 /L |
|
298.3 (13.9-781.0) |
| Platelets >400 x 109 |
|
79 (8.6) |
| Leukocytes x 109/L |
|
8.1 (2.2-33.5) |
| pLeukocytes >10 x 109/L |
|
114 (12.5) |
| Final tumor histology |
| Tumor Grade |
1-2 |
620 (69.4) |
| 3 |
274 (30.6) |
| Histology |
Endometrioid |
735 (82.2) |
| Non-endometrioid |
159 (17.8) |
| LVSI |
Yes |
177 (19.8) |
| No |
717 (80.2) |
| FIGO stage |
Early (I-II) |
806 (90.2) |
| Advanced (III-IV) |
88 (9.8) |
| Lymph node status |
Positive (N1) |
34 (3.8) |
| Negative (N0) |
171 (19.1) |
| Unknown† (Nx) |
689 (77.1) |
| ESGO/ESTRO/ESP risk groups |
Low |
409 (45.7) |
| Intermediate |
159 (17.8) |
| High-intermediate |
162 (18.1) |
| High |
140 (15.7) |
| Advanced/metastatic |
24 (2.7) |
| Adjuvant treatment |
| None |
|
550 (61.5) |
| RT |
VBT |
132 (14.8) |
| EBRT (+/- VBT) |
107 (11.9) |
| CT+CRT |
|
100 (11.2) |
| Other |
|
5 (0.6) |
| Outcome |
| Recurrence |
Yes |
124 (13.9) |
| No |
770 (86.1) |
| Mortality |
Overall |
160 (17.9) |
|
EC-related |
99 (11.1) |
Data is presented in numbers (%) or median (IQR).
Abbreviations: n: Number; FIGO: Federation International Gynecology Obstetric; ESGO: European Society of Gynaecological Oncology; ESTRO: European Society for Radiotherapy and Oncology;
ESP: European Society of Pathology; RT: Radiotherapy; VBT: Vaginal Brachytherapy; EBRT: External beam radiation therapy; CT:
Chemotherapy; CRT: Chemoradiation; EC: Endometrial Cancer.
†no lymphadenectomy performed.
Impact of hematological parameters on response to radiotherapy
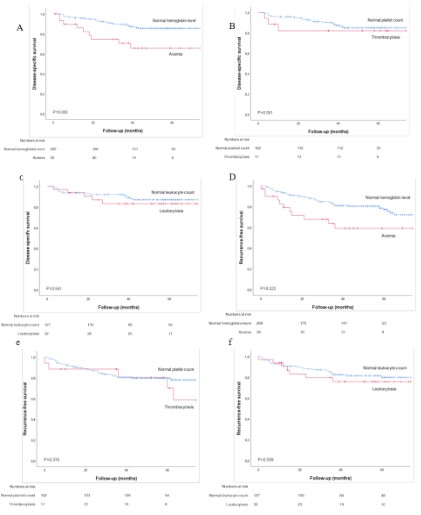
The 5-year DSS and RFS of the preoperative hematological
parameters in all patients who received solely adjuvant RT are
shown in Figure 2A-F. Anemia was associated with a significant
reduced DSS and RFS compared to normal hemoglobin level (respectively, P=0.005 and P=0.025) (Figure 2A,2D). Thrombocytosis
and leukocytosis did not significantly impact the response to RT
(Figure 2B,2C,2E,2F). The 5-year DSS and RFS of the hematological
parameters within patients classified as ESGO/ESTRO/ESP intermediate risk who received solely VBT are shown in Figure S3A-E.
Patients with anemia had a significant decreased DSS compared
to normal hemoglobin level (P=0.041). No significant difference
in DSS and RFS were found for patients with thrombocytosis or
leukocytosis, however numbers were low.
Table 2: Clinicopathological characteristics in relation to hemoglobin-, leukocytes- and thrombocytosis-level.
| hemoglobin (n=103) (n=791) |
Normal |
Anemia |
P |
Normal platelets (n=642) |
Thrombocytosis (n=79) |
P |
Normal leukocytes (n=553) |
Leukocytosis (n=114) |
P |
| Patient characteristics |
| Age |
65.7 (27.2- 91.0) |
68.2 (33.8-93.8) |
0.246 |
66.0 (31.2-93.8) |
64.0 (27.2-90.7) |
0.017* |
66.0 (31.2-93.8) |
65.0 (27.2-86.0) |
0.386 |
| Final tumor histology |
|
|
|
|
|
|
|
|
|
| Tumor grade 1-2 |
560 (70.8) |
60 (58.3) |
0.009* |
448 (69.8) |
52 (65.8) |
0.471 |
383 (69.3) |
81 (71.1) |
0.705 |
| 3 |
231 (29.2) |
43 (41.7) |
|
194 (30.2) |
27 (34.2) |
|
170 (30.7) |
33 (28.9) |
|
| Endometrioid |
656 (82.9) |
79 (76.7) |
0.120 |
531 (82.7) |
62 (78.5) |
0.353 |
455 (81.7) |
94 (82.5) |
0.856 |
| Non-endometrioid |
135 (17.1) |
24 (23.3) |
|
111 (17.3) |
17 (21.5) |
|
101 (18.3) |
20 (17.5) |
|
| LVSI Yes |
149 (18.8) |
28 (27.2) |
0.046* |
121 (18.8) |
23 (29.1) |
0.031* |
109 (19.7) |
26 (22.8) |
0.454 |
| No |
642 (81.2) |
75 (72.8) |
|
521 (81.2) |
56 (70.9) |
|
444 (80.3) |
88 (77.2) |
|
| ESGO/ESTRO/ESP risk groups |
| Low risk |
372 (47.0) |
37 (35.9) |
0.033* |
309 (48.1) |
31 (39.2) |
0.135 |
270 (48.8) |
53 (46.5) |
0.650 |
| Intermediate risk |
146 (18.5) |
12 (11.7) |
0.088 |
105 (16.4) |
7 (8.9) |
0.083 |
77 (13.9) |
17 (14.9) |
0.782 |
| High-intermediate risk |
140 (17.7) |
22 (21.4) |
0.364 |
114 (17.8) |
17 (21.5) |
0.413 |
101 (18.3) |
17 (14.9) |
0.393 |
| High risk |
114 (14.4) |
27 (26.2) |
0.002* |
97 (15.1) |
19 (24.1) |
0.041* |
92 (16.6) |
20 (17.5) |
0.813 |
| Advanced/metastatic |
19 (2.4) |
5 (4.9) |
0.148 |
17 (2.6) |
5 (6.3) |
0.073 |
13 (2.4) |
7 (6.2) |
0.031* |
| Adjuvant treatment |
| None |
|
499 (63.1) |
51 (49.5) |
0.009* |
412 (64.2) |
42 (53.2) |
0.066 |
361 (65.2) |
64 (56.1) |
0.069 |
| RT |
VBT |
122 (15.4) |
10 (9.7) |
0.124 |
87 (13.6) |
3 (3.8) |
0.013* |
58 (10.5) |
15 (13.2) |
0.406 |
|
EBRT (+/- VBT) |
87 (11.0) |
20 (19.4) |
0.012* |
75 (11.6) |
14 (17.7) |
0.124 |
69 (12.5) |
17 (14.9) |
0.480 |
| CT+CRT |
|
79 (10.0) |
21 (20.4) |
0.002* |
65 (10.1) |
19 (24.0) |
<0.001* |
62 (11.3) |
17 (15.0) |
0.260 |
| Other |
|
4 (0.5) |
1 (1.0) |
0.459 |
3 (0.5) |
1 (1.3) |
0.372 |
3 (0.5) |
1 (0.8) |
0.528 |
Data is presented in numbers (%), median (range)
Abbreviations: n: number; LVSI: Lymphovascular Space Invasion; ESGO: European Society of Gynaecological Onoclogy; ESTRO: European Society for Radiotherapy and Oncology; ESP: European
Society of Pathology; RT: Radiotherapy; VBT: Vaginal brachytherapy; EBRT: External beam radiation therapy; CT: Chemotherapy; CRT: Chemoradiation * P<0.05.
Table 3: Cox regression univariable and multivariable analysis of Disease-Specific Survival (DSS) and Recurrence-Free Survival (RFS).
| Variable |
Univariable DSS |
|
Multivariable DSS
Event 66 |
|
Univariable RFS |
|
Multivariable RFS
Event 78 |
|
|
HR (95% CI) |
P value |
HR (95% CI) |
P value |
HR (95% CI) |
P value |
HR (95% CI) |
P value |
| Patient characteristics |
|
|
|
|
|
|
|
|
| Age (continuous) |
1.04 (1.02-1.06) |
<0.001* |
1.03 (1.00-1.06) |
0.009* |
1.04 (1.01-1.05) |
<0.001* |
1.03 (1.00-1.05) |
0.022* |
| Hematological parameters |
|
|
|
|
|
|
|
|
| Anemia |
3.19 (2.02-5.02) |
<0.001* |
2.31 (1.19-4.50) |
0.013* |
2.32 (1.49-3.60) |
<0.001* |
1.71 (0.91-3.19) |
0.091 |
| Thrombocytosis |
1.90 (1.08-3.34) |
0.025* |
1.06 (0.49-2.30) |
0.872 |
1.31 (0.73-2.36) |
0.363 |
0.70 (0.32-1.55) |
0.382 |
| Leukocytosis |
1.65 (0.95-2.86) |
0.074 |
1.37 (0.74-2.55) |
0.312 |
1.45 (0.85-2.44) |
0.168 |
1.53 (0.84-2.74) |
0.159 |
| ESGO/ESTRO/ESP risk groups |
|
|
|
|
|
|
|
|
| Low |
1 |
|
1 |
|
1 |
|
1 |
|
| Intermediate |
7.59 (3.01-19.12) |
<0.001* |
3.06 (0.98-9.53) |
0.053 |
6.20 (3.06-12.55) |
<0.001* |
2.38 (0.88-6.39) |
0.087 |
| High-intermediate |
3.90 (1.38-10.96) |
0.010* |
1.59 (0.44-5.65) |
0.472 |
5.23 (2.56-10.89) |
<0.001* |
3.43 (1.44-8.16) |
0.005* |
| High |
32.66 (13.99-76.23) |
<0.001* |
18.3 (7.64-43.94) |
<0.001* |
22.21 (11.64-42.37) |
<0.001* |
16.78 (8.05-34.83) |
<0.001* |
| Advanced/metastatic 101.97
(40.33-257.79) |
|
<0.001* |
72.1 (27.36-189.97) |
<0.001* |
36.59 (15.79-84.74) |
<0.001* |
33.68 (13.15-86.29) |
<0.001* |
Abbreviations: DDS: Disease-specific survival; RFS: Recurrence-free survival; HR: Hazard ratio; CI: Confidence interval; ESGO: European Society of
Gynaecological Onoclogy; ESTRO: European Society for Radiotherapy and Oncology; ESP: European Society of Pathology, *P<0.05.
Discussion
In this study, the prognostic and predictive relevance of preoperative abnormal hematological parameters in patients with EC
was evaluated. Anemia was identified as an independent prognostic factor for DSS, along with age and ESGO/ESTRO/ESP ‘high- and advanced/metastatic’ risk. Furthermore, anemia seemed an
overall predictive factor for response to adjuvant RT, and specifically for patients with ESGO/ESTRO/ESP intermediate risk who
received solely VBT.
Although most patients with EC present with postmenopausal bleeding as an early symptom, this rarely causes anemia at
diagnosis. Hence, the development of cancer-related anemia
in EC is more likely caused by inflammatory cytokines which results in a shortened survival of red blood cells, suppression of
erythroid progenitor cells, impaired iron utilization, and inadequate Erythropoietin (EPO) production [7,25]. Anemia in patients
with an absolute or relative EPO deficiency seems to be more
aggressive in solid tumors [26]. Therefore, it is suggested that
preoperative anemia in EC could be a biomarker of tumor burden
and/or aggressive tumor behavior [25,26]. In our study cohort we
observed that patients with anemia were significantly more often
allocated to ESGO/ESTRO/ESP high risk group, grade 3 EC, and the
presence of LVSI. In both univariable and multivariable DSS analysis, we found anemia as independent prognostic factor. To our
knowledge, the presence of anemia has so far not been related
to the ESGO/ESTRO/ESP risk groups. Previous studies did show
a significantly higher prevalence of anemia in patients classified
into the ESGO/ESTRO/ESP high risk group; FIGO advanced-stage,
grade 3 EC and LVSI [16]. The 5-year RFS was significantly reduced
in patients with anemia compared to those without anemia.
However, anemia was not an independent prognostic factor for
the RFS, comparable to the findings of Wilairat et al [27].
Cancer-related anemia may also cause tumor hypoxia, which
may lead to a reduced response to RT [4,17-19]. Normally, hypoxia will lead to an EPO increase, however due to the cancer-associated inflammation the EPO production is insufficient and the
iron metabolism is impaired. VBT is given for local control of the
tumor and EBRT could be applied to control locoregional recurrence [19]. In patients within our study, who received RT and even
with solely VBT within the ESGO/ESTRO/ESP intermediate risk
group, anemia was correlated with a significantly reduced DSS.
However, numbers were low and therefore multivariable analysis
was not achievable. So far, no other studies including EC patients
have been performed to compare our findings.
Three recent meta-analyses published the clinicopathological
and/or prognostic significance of preoperative thrombocytosis in
EC [8,9,13]. In line with our findings, a significant association of
thrombocytosis with FIGO advanced-stage, LVSI and grade 2-3 EC
was found [8,13]. The prognostic relevance, however, still remains
conflicting in EC studies, probably due to different used cut-off values for thrombocytosis [8,9,13]. Comparable to our study, Njolstad et al. found a significant reduced DSS of patients with thrombocytosis [11]. However, thrombocytosis as dichotomous value
instead of continuous platelet count was not found as independent factor for DSS and RFS [8]. The pathophysiological mechanism between tumor behavior and preoperative thrombocytosis
is not fully elucidated [13]. The overexpression of inflammatory
cytokines results in an increase of megakaryocyte maturation
which causes increased platelet production [28]. Some hypothesize that platelets infiltrate tumor tissue and contribute to tumor
growth by secreting pro-angiogenic factors and pro-tumorigenic
factors, while others suggest a plateletcancer interaction facilitating cancer cell migration, which contributes cancer metastasis
[29].
The impact of leukocytosis on tumor behavior may also be
explained by upregulation of inflammatory cytokines and hematopoietic growth factor through tumor cells, thus promoting
enhanced inflammation, leukocytosis, angiogenesis and tumor cell proliferation [6,30]. We observed a significant association
between leukocytosis and the ESGO/ESTRO/ESP advanced/metastatic risk group in our study cohort, however leukocytosis was
not significant in univariable and multivariable analysis. A recent
meta-analysis found a correlation between leukocytosis and FIGO
advanced-stage [15], of whom only one study performed a multi-variable analysis for RFS with comparable results as our study [14].
Due to the pro-angiogenic factors induced with elevated platelet and leukocyte count, its suspected that angiogenesis will lead
to a better drug or oxygen access to tumor cells, however there
is a lack of homogeneity of vasculature density in different parts
of the same tumor which could affect outcome and response to
adjuvant treatment [4]. Although we did not observe impact of
thrombocytosis and/or leukocytosis on response to RT, included
numbers were low. In patients with cervical cancer leukocytosis
was related to poor response to RT, but due to differences in carcinogenesis it may be difficult to compare those results with EC [5].,/p>
There are some limitations inherent to the retrospective design. First, adjuvant treatment was not uniformly applied which
could lead to differences in outcome. Second, due to the fact
that most of our labs do not run routine complete blood count,
platelet- and leukocyte count were not available for all included
patients. Finally, complete molecular data according The Cancer Genome Atlas is not available for the patients in this cohort.
However, within a subset of the PIPENDO cohort, we do have immunohistochemistry of p53 and mismatch repair proteins. Within
patients with p53-abnormal, anemia was associated with significant reduced DSS and RFS compared to patients with normal hemoglobin (data not shown).
To our knowledge, this is the first study that addressed the
relationship of all three, often routinely obtained, preoperative
abnormal hematological parameters with clinicopathological
characteristics and univariable and multivariable outcome in EC.
Other strengths of this study includes its multicenter design resulting in the largest patient cohort to date, and a well-documented
and long follow-up period.
Future studies in a prospective study design, may determine
the prognostic and/or predictive value of preoperative abnormal
hematological markers (more specific anemia) in addition to the
molecular markers in EC. When confirmed, studies should explore
in more detail the cause between for example anemia and impaired prognosis.
Conclusion
Our data demonstrated the independent prognostic impact
of preoperative anemia in patients with EC. In addition, anemia
seems to be associated as predictive biomarker for response to
radiotherapy. It remains unclear whether preoperative anemia reflects tumor aggressiveness or reduced response to radiotherapy.
So, prospective validation in a larger study cohort is needed to
verify anemia as predictive biomarker for radiotherapy.
Conflict of interest: The authors have declared no conflicts of
interest.
Funding: This work was not funded.
References
- Bokhman JV. Two pathogenetic types of endometrial carcinoma.
Gynecol Oncol. 1983; 15: 10-17.
- Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, et al.
ESGO/ESTRO/ESP guidelines for the management of patients with
endometrial carcinoma. Int J Gynecol Cancer. 2021; 31: 12-39.
- Reijnen C, Gogou E, Visser NCM, Engerud H, Ramjith J, et al. Preo-perative risk stratification in endometrial cancer (ENDORISK) by
a Bayesian network model: A development and validation study.
PLoS Med. 2020; 17: e1003111.
- Koukourakis MI, Giatromanolaki A, Sivridis E, Fezoulidis I. Cancer
vascularization: implications in radiotherapy? Int J Radiat Oncol
Biol Phys. 2000; 48: 545-553.
- Cho Y, Kim KH, Yoon HI, Kim GE, Kim YB. Tumor-related leukocytosis is associated with poor radiation response and clinical outcome
in uterine cervical cancer patients. Ann Oncol. 2016; 27: 2067-2074.
- Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev.
2005;14: 2840-2847.
- Birgegård G, Aapro MS, Bokemeyer C, Dicato M, Drings P, Hornedo
J, et al. Cancer-related anemia: pathogenesis, prevalence and
treatment. Oncology. 2005; 68: 3-11.
- Nie D, Yang E, Li Z. Pretreatment thrombocytosis predict poor
prognosis in patients with endometrial carcinoma: a systematic
review and meta-analysis. BMC Cancer. 2019; 19: 73.
- Ye Q, Wu Z, Xia T, Liu D, Yang Y, et al. Pre-treatment thrombocytosis
predicts prognosis of endometrial cancer: A meta-analysis of 11
studies. Exp Ther Med. 2020; 19: 359-366.
- Worley MJ, Nitschmann CC, Shoni M, Vitonis AF, Rauh-Hain JA, et
al. The significance of preoperative leukocytosis in endometrial
carcinoma. Gynecol Oncol. 2012; 125: 561-565.
- Njolstad TS, Engerud H, Werner HM, Salvesen HB, Trovik J. Preo-perative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecol Oncol. 2013; 131: 410-415.
- Tamussino KF, Gucer F, Reich O, Moser F, Petru E, et al. Pretreatment hemoglobin, platelet count, and prognosis in endometrial
carcinoma. Int J Gynecol Cancer. 2001; 11: 236-240.
- Bai YY, Du L, Jing L, Tian T, Liang X, Jiao M, et al. Clinicopathological and prognostic significance of pretreatment thrombocytosis in
patients with endometrial cancer: a meta-analysis. Cancer Manag
Res. 2019; 11: 4283-4295.
- Salem H, Abu-Zaid A, Aloman O, Abuzaid M, Alsabban M, et al.
Preoperative Leukocytosis as a Prognostic Marker in Endometrioid-Type Endometrial Cancer: A Single-Center Experience from
Saudi Arabia. Gulf J Oncolog. 2020; 1: 51-58.
- Abu-Zaid A, Alomar O, Baradwan S, Abuzaid M, Alshahrani MS, et
al. Preoperative leukocytosis correlates with unfavorable pathological and survival outcomes in endometrial carcinoma: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol.
2021; 264: 88-96.
- Abu-Zaid A, Alomar O, Abuzaid M, Baradwan S, Salem H, Al-Badawi
IA. Preoperative anemia predicts poor prognosis in patients with
endometrial cancer: A systematic review and metaanalysis. Eur J
Obstet Gynecol Reprod Biol. 2021; 258: 382-390.
- Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol.
2004; 43: 396-403.
- Durand RE. Keynote address: the influence of microenvironmental
factors on the activity of radiation and drugs. Int J Radiat Oncol
Biol Phys. 1991; 20: 253-258.
- Moon EJ, Petersson K, Olcina MM. The importance of hypoxia in
radiotherapy for the immune response, metastatic potential and
FLASH-RT. Int J Radiat Biol. 2022; 98: 439-451.
- Visser NC, Bulten J, van der Wurff AA, Boss EA, Bronkhorst CM,
Feijen HW, et al. PIpelle Prospective ENDOmetrial carcinoma (PI-PENDO) study, pre-operative recognition of high risk endometrial
carcinoma: a multicentre prospective cohort study. BMC Cancer.
2015; 15: 487.
- Bouwman F, Smits A, Lopes A, Das N, Pollard A, et al. The impact
of BMI on surgical complications and outcomes in endometrial
cancer surgery--an institutional study and systematic review of the
literature. Gynecol Oncol. 2015; 139: 369-376.
- Gynaecologie WO. Landelijke richtlijn endometriumcarcinoom
2018.
- Sundar S BJ, Crosbie E, Drake A, Edmondson R, Fotopoulou C, et al.
BGCS Uterine Cancer Guidelines: Recommendations for Practice
2017 [23-6-2021].
- McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide
prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009; 12: 444-454.
- Moliterno AR, Spivak JL. Anemia of cancer. Hematol Oncol Clin
North Am. 1996; 10: 345-363.
- Obermair A, Handisurya A, Kaider A, Sevelda P, Kolbl H, et al. The
relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients: a prospective review.
Cancer. 1998; 83: 726-731.
- Wilairat W, Benjapibal M. Presence of anemia and poor prognostic
factors in patients with endometrial carcinoma. Asian Pac J Cancer
Prev. 2012; 13: 3187-3190.
- Berridge MV, Fraser JK, Carter JM, Lin FK. Effects of recombinant
human erythropoietin on megakaryocytes and on platelet production in the rat. Blood. 1988; 72: 970-977.
- Li N. Platelets in cancer metastasis: To help the «villain» to do evil.
Int J Cancer. 2016; 138: 2078-2087.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454: 436-444.