Introduction
Brain metastasis is a common complication in lung cancers and
represent a negative prognostic factor. Therapeutic options for patients with BM are largely palliative and include surgical resection,
whole-brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), or their combinations, while chemotherapy is rarely used
due to its limitation to effectively cross the blood-brain barrier [1].
These treatments often leave patients with adverse neurocognitive function, poor quality of life, and dismal prognosis [2].
Although immune checkpoint inhibitors have revolutionized
treatment landscape for patients with non-small cell lung cancers
(NSCLC) - influences on brain metastases (BM) are still uncertain
due to the fact that these patients have generally been excluded
from clinical trials or have been underrepresented.
Having in mind that immunologic microenvironment of metastatic disease can vary by specific organ, there is possible impact
on the response to immunotherapy, and prognosis as well.
Our research aims to investigate a possibility of a link between
metastases site and immunotherapy response.
Patients and methods
The study included a total of 141 patients with pathologicaly/
cytologically confirmed advanced NSCLC treated with ICI monotherapy in first line between June 2017. to June 2021. Study was
conducted at Clinic for pulmonology, University Clinical center
of Serbia. Eligible patients were ≥18 years and Eastern Cooperative Oncology Group (ECOG) performance status was ≤1. Patients
treated with Pembrolizumab monotherapy were required to have
a programmed death ligand 1 (PD-L1)-positive tumor with TPS
≥50% and they received pembrolizumab 200 mg every 3 weeks.
Abscence of anaplastic lymphoma kinase (ALK) and epidermal
growth factor receptor (EGFR) mutations was mandatory.
Tumor lesions were measured using computed tomography at
baseline and every 9 weeks thereafter. Tumor size was recorded as
the sum of the longest diameters (SLD) assessed per RECIST v1.1
by independent central review. PD-L1 expression was assessed in
contemporaneous biopsy samples using immunohistochemistry.
Patients were divided into two subgroups according to the presence of CNS metastases, based on which subgroup comparison
was performed. All patients with BM, who were enrolled in our
study, received stereotactic radiotherapy (SRS) or whole brain radiotherapy (WBRT) in addition to ICI.
Descriptive statistics were reported as frequencies and percentages for categorical variables, and medians, standard deviations
from the mean (SD), and ranges for continuous variables. Hazard
ratios were estimated using the Cox proportional-hazards model.
Kaplan-Meier curves were used to estimate mean progression-free survival (PFS) and mean overall survival (OS). Confidence
interval of 95% was used for medians and they were calculated
using bootstrapping. For testing hypotheses, p-value of <0.05 was
considered as significant. Statistical analyses were done using the
IBM SPSS ver. 26 software (IBM Corporation, USA).
Results
Overall 141 metastatic NSCLC patients treated with ICI were
enrolled in this study. Among them, 84 (59.6%) were men and
57 (40.4%) were women. Median age was 63 [standard deviation
(SD) 8,720, range 35-89] years. There were 72 (51.1%) current
smokers, 54 (38.3%) former smokers, and 15 (10.6%) never-smokers. The dominant tumor histology was non-squamous [66.0%].
The most frequent metastatic site was lung, detected in 43 patients (30.5%) and followed up by CNS (19.9%). Pleura, bone and
liver metastases were detected in 14.2%, 12.8% and 9.9% of patients, respectively.
The baseline clinical and demographic characteristics are reported in Table 1.
The median PFS for all patients was 10 months, median OS was
14 months. Patients were divided into two subgroups: one group
included patients with CNS metastases and other included patients without CNS metastases, but with other metastases. ICI significantly prolonged PFS in group with CNS metastases with median of PFS: 11.5 months [95% CI: 8-15 months] versus 9 months
[95% CI: 7-13 months] in the group without CNS metastases (HR=
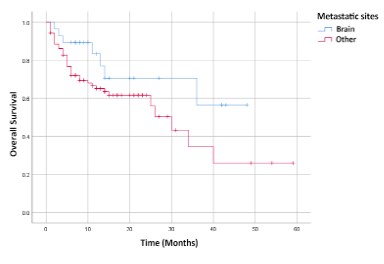
0.416, 95% CI: 0.19-0.91, p 0.028) as shown in Figure 1. There
was no statistically significant improvement in OS in the group of
patients with CNS metastases (median OS: 11,5 months [95% CI:
8-15 months] versus 14 months [95% CI: 11-15.97 months] compared to a group of patients without baseline CNS metastases;
HR= 0.531, 95% CI: 0.24-1.17, p 0.116 (Figure 2).
Table 1: Baseline characteristics and demographics.
| Characteristics |
CNS metastases |
Other metastatic sites |
| Age (range) |
63.5 (43-81) |
63 (35-89) |
| Sex |
|
|
| Female (%) |
14(50) |
43(38.1) |
| Male (%) |
14(50) |
70(61.9) |
| Histology |
|
|
| Non-squamous (%) |
20 (71.4) |
73 (64.8) |
| Squamous (%) |
1 (3.6) |
26 (23) |
| NSCLC NOS (%) |
7 (25) |
14 (12.4) |
| PDL1 expression |
|
|
| PDL1 ≤ 60% |
10 (15.4) |
55 (84.6) |
| PDL1 61%-80% |
7 (16.7) |
35 (83.3) |
| PDL1 > 80% |
11 (32.4) |
23 (87.6) |
| Smoking status |
|
|
| Current smokers |
18 (12.8) |
54 (38.3) |
| Former smokers |
9 (6.4) |
45 (31.9) |
| Never smokers |
1 (0.7) |
14 (9.9) |
| Pack-years (range) |
40 (0-160) |
40 (0-100) |
Discussion
Some retrospective cohort studies suggest that liver, bone,
and brain metastases in patients receiving immunotherapy lead
to a significant association with worse PFS and OS compared with
other sites [3,4].
It is well known that the immunologic microenvironment of
metastatic disease can vary by specific organ, with a possible
impact on the response to immunotherapy, as well as prognosis
[3,5]. For instance, lymphocyte population of the liver is selectively enriched with natural killer (NK) and T cells, which is critical
for first-line immune defense against invading pathogens, modulation of liver injury and recruitment of circulating lymphocytes
[6]. In the brain, the blood–brain barrier and brain-resident cell
types (e.g., microglia) cause an immunosuppressive microenvironment [7].
Brain metastases (BM) occur in 20% to 32% of patients diagnosed with non–small cell lung cancer and generally represent
a negative prognostic factor for patients with solid malignancies
[8-10]. However, among non-oncogene NSCLC patients with BM
there are limited data available on intracranial efficacy of immunotherapy because these patients have generally been excluded
from clinical trials or are underrepresented [11].
In the majority of cases BM are approached with locoregional
treatments, due to the fact that blood–brain barrier limits the efficacy of some systemic drugs [12]. The mechanism of action of
ICIs is based on altered immune cell activity rather than direct
action of these agents in the brain [13]. The presence of tumor
infiltrating lymphocytes (TILs) and the expression of PD-L1 have
been observed in brain metastases from patients with NSCLC and
it has been shown that PD-L1 expression is lower in BM compared
with the primary tumor [14]. In addition, the administration of ICI
in patients with BM may be associated with pseudoprogression
and subsequent symptom aggravation due to increased edema
before the tumor actually shrinks [11]. This phenomenon may
necessitate symptomatic treatment with corticosteroids which
could affect the treatment potency.
As shown previously in our study, ICI significantly prolonged
PFS in the group with brain metastases compared to a group of
patients with other metastases, while there was no statistically
significant improvement in the same group OS.
Considering all the above how could we explain that ICI significantly prolonged PFS in group of patients with brain metastases
compared with those with other metastases?
All patients with BM, who were included in our study, received
stereotactic radiotherapy (SRS) or whole brain radiotherapy
(WBRT) in addition to ICI. Therefore, an abscopal effect provides a
sound potential rationale for our results.
Many study results suggest that localized radiotherapy , traditionally used to control localized disease, not only directly kills
tumor cells but also may elicit an immune response by promoting
the cross-priming of tumor-specific CD8 T cells, that attack both
irradiated and distant, nonirradiated tumors [15-17]. The RT-induced antitumor T cell response can be enhanced by combination
with ICI [18]. The combination of radiation and immunotherapy
may increase the occurrence of abscopal effect, [19-21] with rates ranging from 25% to 52% with immune checkpoint inhibitors [19,21].
The results of a retrospective study by Min Wu et al. are consistent with ours. The study included patients with advanced
NSCLC who had received radiotherapy for a primary or metastatic
solid tumor. They aimed to determine the differences in systemic
immune activation after RT to the brain, bone, lung, liver, adrenal gland, and soft tissue during immunotherapy synchronously.
Study concluded that irradiation to brain had the strongest effect
on immune activation and response to immunotherapy treatment
in advanced NSCLC. They assumed that this may be due to the fact
that the blood–brain barrier was breached with RT [22]. A metaanalysis conducted by Wenjing Li et al also suggested that PD-1 or
PD-L1 inhibitors can reduce the risk of both disease progression
and death of patients with brain metastases of NSCLC, who have
been pretreated with local therapies and/or in whom the brain
lessions are asymptomatic [23].
Conclusion
Our study found that brain metastases in patients with stage
IV NSCLC with PD-L1 expression ≥50% responded best to immunotherapy.
Given the fact that all patients received radiotherapy in addition to ICI, it is crucial to highlight the effects of the synergistic
action of these two therapies. Further clinical trials are needed
to define the role of immunotherapy in NSCLC patients with BM.
Declarations
Conflict of interest: The authors declare that they have no
conflict of interest.
Financial disclosures: The authors declare that no funds,
grants, or other support were received during the preparation of
this manuscript.
Ethical statement: The authors received ethical approval for
the study from the ethical board and all patients signed ICF.
Funding: None
Acknowledgements: None
References
- Di Giacomo, A., Valente, M., Cerase, A. et al. Immunotherapy of
brain metastases: breaking a “dogma”. J Exp Clin Cancer Res. 2019;
38: 419.
- Pathak R, Amini A, Hill A, Massarelli E, Salgia R. Immunotherapy
in Non-Small Cell Lung Cancer Patients with Brain Metastases: Clinical Challenges and Future Directions. Cancers (Basel). 2021; 13:
3407.
- Botticelli A, Cirillo A, Scagnoli S. et al. The Agnostic Role of Site of
Metastasis in Predicting Outcomes in Cancer Patients Treated with
Immunotherapy. Vaccines. 2020; 8: 203.
- Bilen MA, Shabto JM, Martini DJ, Liu Y, et al. Sites of metastasis
and association with clinical outcome in advanced stage cancer
patients treated with immunotherapy. BMC Cancer. 2019; 19.
- Obenauf AC, Massagué J. Surviving at a Distance: Organ-Specific
Metastasis. Trends in Cancer. 2015; 1: 76–91.
- Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its
role in inflammation and homeostasis. Cell Mol Immunol. 2016;
13: 267-76.
- Quail DF, Joyce JA. The Microenvironmental Landscape of Brain Tumors. Cancer Cell. 2017; 31: 326-341.
- Barnholtz-Sloan, Jill S., et al. «Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan
Detroit Cancer Surveillance System.» Journal of clinical oncology.
2004; 22: 2865-2872.
- Molinier O, Audigier-Valette C, Cadranel J, Monnet I, Hureaux J,
et al. OA 17.05 IFCT1502 CLINIVO: Real-Life Experience with Nivolumab in 600 Patients (Pts) with Advanced Non-Small Cell Lung
Cancer (NSCLC). J Thorac Oncol. 2017; 12: S1793.
- Sperduto PW, Kased N, Roberge D, Xu Z, et al. Summary Report on
the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients With Brain
Metastases. Journal of Clinical Oncology. 2012; 30: 419-425.
- El Rassy E, Botticella A, Kattan J, Le Péchoux C, Besse B, et al. Non-small cell lung cancer brain metastases and the immune system:
From brain metastases development to treatment. Cancer Treatment Reviews. 2018; 68: 69-79.
- Eguren-Santamaria I, Sanmamed MF, Goldberg SB, et al. PD-1/PD-L1 blockers in NSCLC brain metastases: challenging paradigms and
clinical practice. Clinical Cancer Research, clincanres. 2020.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012; 12: 252-264.
- Mansfield AS, Aubry MC. et al. Temporal and spatial discordance
of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Annals of Oncology. 2016; 27: 1953-1958.
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on
the abscopal effect. Current Problems in Cancer. 2016; 40: 25-37.
- Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint
blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015; 16: e498-e509.
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of
distant untreated tumors (abscopal effect) is immune mediated.
Int J Radiat Oncol Biol Phys. 2004; 58: 862-870.
- Park SS, Dong H, Liu X, et al. PD-1 restrains radiotherapy-induced
abscopal effect. Cancer Immunol Res. 2015; 3: 610-619.
- Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of
radiotherapy on advanced melanoma patients who progressed
after ipilimumab immunotherapy. Oncoimmunology. 2014; 3:
e28780-1-e28780-9.
- Chandra RA, Wilhite TJ, Balboni TA, et al. A systematic evaluation
of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology.
2015; 4: e1046028-1-e1046028-7.
- Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2—tumor and immunological responses. Sci Transl Med. 2012; 4: 137ra74-1–137ra74-1-7
- Wu M, Liu J, et al. Systemic Immune Activation and Responses of
Irradiation to Different Metastatic Sites Combined With Immunotherapy in Advanced Non-Small Cell Lung Cancer. Front Immunol.
2021; 12: 803247.
- Li W, Jiang J, Huang L, Long F. Efficacy of PD-1/L1 inhibitors in brain
metastases of non-small-cell lung cancer: pooled analysis from seven randomized controlled trials. Future Oncol. 2022; 18: 403-412.