Introduction
Wound healing is a complex process that occurs in three phases
that can be categorized as inflammation, proliferation and re-modeling or scar formation [1,2]. Wound closure is mediated by the
proliferation of fibroblasts, which secrete collagen types I and III
[3]. However, excessive fibroblast proliferation results in overproduction of collagen, which is a possible cause of Hypertrophic Scar
(HS) or keloid formation [4]. HS formation results from dysregulation of the wound healing process [5,6]. Factors such as inflammation, collagen accumulation, and reduced fibroblast apoptosis
or skin mechanical tension as well as excessive contraction at the
wound site have been implicated in the formation of HS, although
the mechanism remains to be fully elucidated [7-9]. Donkey skin
contains large amounts of collagen secreted by fibroblasts. In our
preliminary experiment, we showed that HS formation occurs in
donkey skin, while keloid production does not. The mechanism by
which donkey skin suppresses excess collagen secretion in wound
healing remains unclear.
Transforming growth factor-β1 (TGF-β1) has been widely reported to play an essential role in wound healing [10,11]. Furthermore, enhanced TGF-β1 expression stimulates type I and type III
collagen synthesis in fibroblasts by activating the Smad2/3 signaling pathway, resulting in HS formation [12]. Therefore, TGF-β1 is
a key target for the development of novel therapeutic strategies
for HS [13].
5-Fluorouracil (5-FU) is a derivative of uracil in which the hydrogen at position five is replaced by fluorine. This anti-metabolic
drug inhibits the biochemical activity of rRNA and mRNA in the nucleus, as well as the synthesis of DNA and proteins, leading to cell
damage and death. 5-FU exerts its cytotoxic effects at all stages of
cell proliferation, and also inhibits collagen synthesis [14]. It can
also inhibit the growth of tumours and normal tissues [15]. 5-FU is
widely used as a chemotherapeutic agent because of its capacity
to induce apoptosis in malignant cells [16]. Subconjunctival 5 -FU
injections administered after glaucoma surgery led to apoptotic
cell death in the conjunctival epithelium [17,18].
In this study, we investigated the ability of 5-FU to inhibit excessive collagen secretion and reduce HS formation in donkey skin
wound to provide a theoretical basis for the development of strategies to achieve scar-free wound healing.
Materials and methods
Donkeys
In this study, we used six female donkeys (aged 2–4 years;
average weight, 200–230 kg). Before the experiment, all donkeys
underwent a health examination to confirm the absence of systemic disorders or lameness that could hinder wound healing. The
donkeys were fed 0.8 kg concentrate and allowed free access to
hay and water.
Wound generation and wound images
Each donkey was anesthetized by intraperitoneal injection
with 10% chloral hydrate (4 mL/kg) before dorsum shaving and
application of antiseptic. Six full-thickness skin wounds (diameter
1.5 cm) were created with a metal template on both sides of the
back midline of each donkey (three wounds on each side) with a
minimum 50 cm space between each wound. The donkeys were
then allocated randomly to the control or the 5-FU (Choitec Pharmaceuticals Co., Ltd. Hainan, China) treatment groups (n = 3 per
group). On days 4, 5, 6, 7, 9, 11, 13 and 15 after wound creation,
6 mL 5-FU liquid (25 mg/mL) was applied topically and injected
in two separate sites around each wound; control wounds were
treated with 10 mL saline. Wound images were captured using a
camera on days 0, 7, 17, 30, 40 and 100, with a ruler included in
the image for reference. The wound healing areas were analyzed
using Image J software and the wound healing rate was calculated according to the following formula:Wound healing rate (%) =
[(area on day 0−area on day n)/area on day 0] × 100%.
Skin biopsy collection and staining observation
On days 0, 7,17 and 100, a full-thickness skin sample (diameter
2 cm) was collected from the wound margins and including 0.25
cm of normal skin. Each sample was equally divided into two parts.
One was immediately frozen in liquid nitrogen for qRT-PCR analysis, and the other was immediately placed in 10% formaldehyde and store for 2 weeks prior to histological staining. The histological samples were embedded in optimum cutting temperature
compounds, and sectioned (5μm thickness) on a microtome. The
sections were stained with Hematoxylin and Eosin (HE) to evaluate the morphological features of the tissues and skin thickness,
and with Masson’s trichrome (MS) to evaluate the fibrous connective tissues after wound healing. Histological examinations were
conducted under a microscope. The epidermis thickness and collagen density were determined with Image J software.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from samples using TRIzol reagent
(Invitrogen company, Shanghai, China). The RNA samples were
quantified, and reverse transcribed into first-strand cDNA using
transcriptor first strand cDNA synthesis Kit (Roche, Basel, Switzerland). Each reaction was carried out in volumes of 20 μL, which
included 10 μL Tip Green qPCR Super Mix, 1 μL of each primer,
and 1 μL cDNA template. The PCR amplification conditions were
as follows: 95°C for 10 min, followed by 45 cycles of 95°C for 30
s, target gene annealing temperature for 30 s, and 72°C for 30 s.
The following primers were used in this study: TGF-β1-F: 5’-ATTCCTGGCGCTACCTCAGT-3’, and R: 5’-CGCACGACTCCAGTGACATC-3’;
Smad3-F: 5’-AGAGACCAGCGACCACCAGATG-3’, and R: 5’-GCTGCGAGGCGTGGAATGTC-3’; Smad7-F: 5’-CTCGGAAGTCAAGAGGCTGTGTTG-3’, and R: 5’-TCTAGTTCGCAGAGTCGGCTAAGG-3’; COL1A1-F: 5’-GCAACGTGTTGTGCGATGAC-3’, and R: 5’-CGACTCCTGTGGTTTGGTCG-3’; COL1A2-F: 5’-GCTGGTAGTCGTGGTGCAACTG-3’,
and R: 5’-TTGGTCCAGGTCTGCCGTCTATAC-3’; COL1A3-F: 5’-TGCTGCTGGTACTCCTGGTCTG-3’, and R: 5’-CACCTGCACTGCCTGGTTCAC-3’; β-actin-F: 5’-CGACATCCGTAAGGACCTGT-3’, and R:
5’-CAGGGCTGTGATCTCCTTCT-3’. Relative gene expression was
normalized against internal controls and calculated using the ΔΔCT
method.
Enzyme-linked immunosorbent assay (ELISA)
Protein levels of TGF-β, COL1A1, and COL1A2 in donkey skin
wound samples were analyzed using specific ELISA kits (Shanghai
Enzyme Union Biotechnology Co. Ltd, Shanghai, China). The absorbance at 450 nm (OD450) was measured using a microplate
reader (Thermo Fisher Scientific).
Statistical analysis
All statistical analyses were performed using SPSS 19.0. Data
were presented as the mean ± standard deviation and analyzed by
one-way ANOVA. P < 0.05 was set as the threshold for statistical
significance.
Result
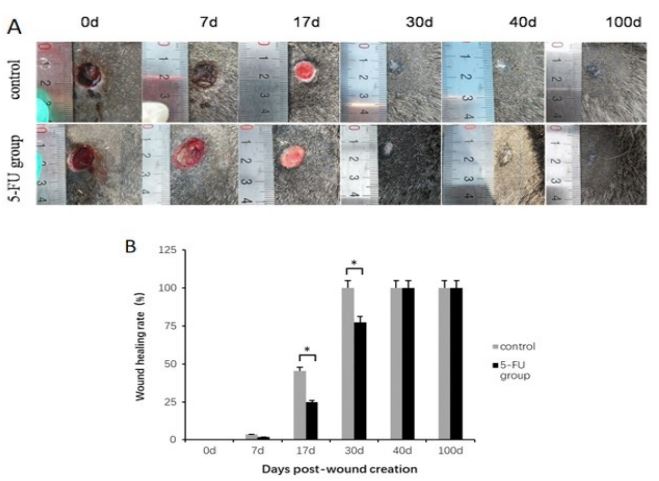
Macroscopic Evaluation
Wound healing occurred gradually over time (Figure 1A). On
day 7, the wounds started to shrink due to re-epithelialization at
the wound edge. Before day 30 post-wounding, healing occurred
more slowly in the 5-FU treatment group than that in the control.
By day 100, there was no HS formation in the 5-FU injection group,
while slight HS formation was observed in the control group.
Compared with the control group on days 17 and 30, wound
healing was significantly slower in the 5-FU group (Figure 1B).
However, after day 40, there were no significant differences in the wound healing rate of the 5-FU and control groups.
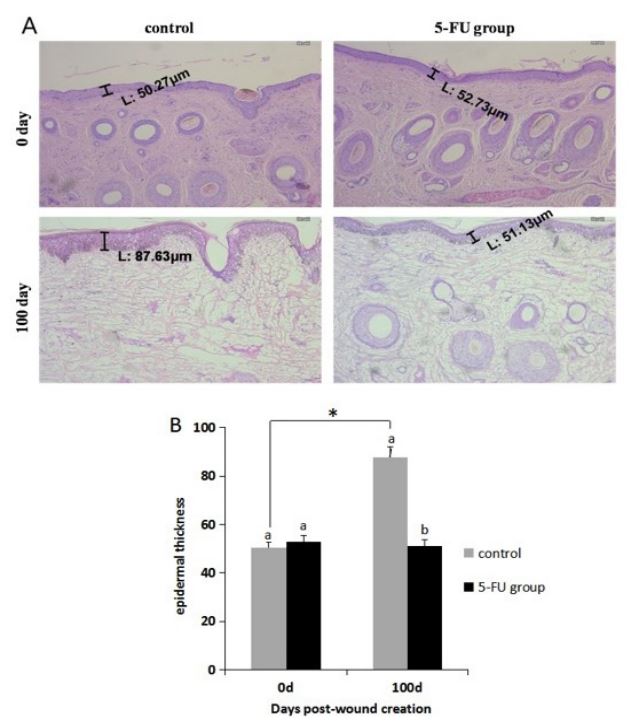
The epidermal thickness
Skin samples collected on days 0 and 100 post-wound creation
were stained with HE to determine the epidermal thickness (Figure 2). In the 5-FU group, there was no significant difference in
the epidermal thickness of samples collected on day 0 and 100. In
contrast, the epidermal thickness in the control group was significantly increased on day 100 compared with that on day 0.
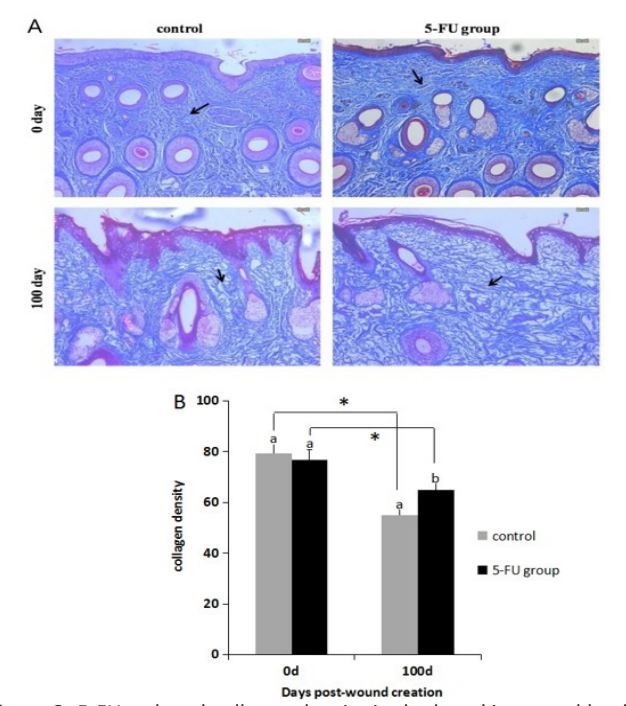
Collagen deposition
MS staining showed the presence of coarse collagen bundles in
the reticular dermis of the 5-FU and control groups (Figure 3). In
all wounds, the collagen bundles were distributed more sparsely
on day 100 compared with 0. Furthermore, on day 100, the collagen fibers were more ordered, with wider gaps, less deposition
and lower density in the 5-FU group compared with the control
group.
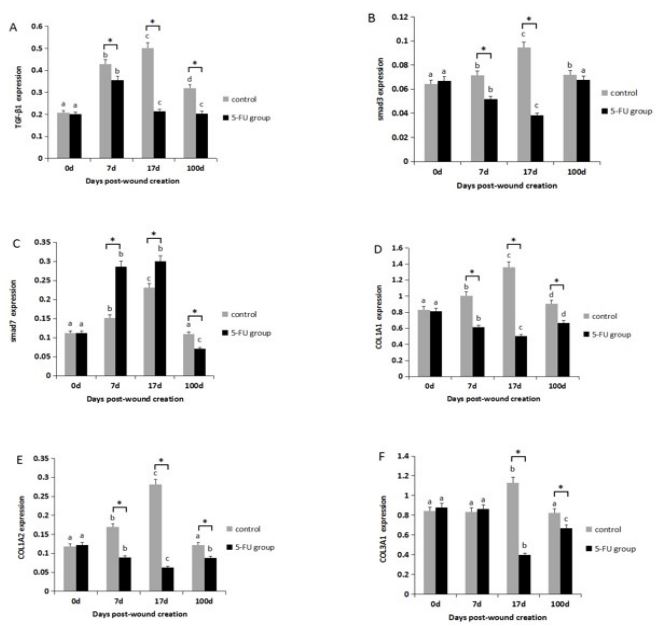
Collagen synthesis-related gene expression
Compared with the control group, gene expression of TGF-β1,
Smad3, COL1A1, COL1A2 and COL3A1 was significantly reduced
in the 5-FU group on days 7 or 17 (P < 0.05), while the opposite
pattern of expression was observed for Smad7 (Figure 4).
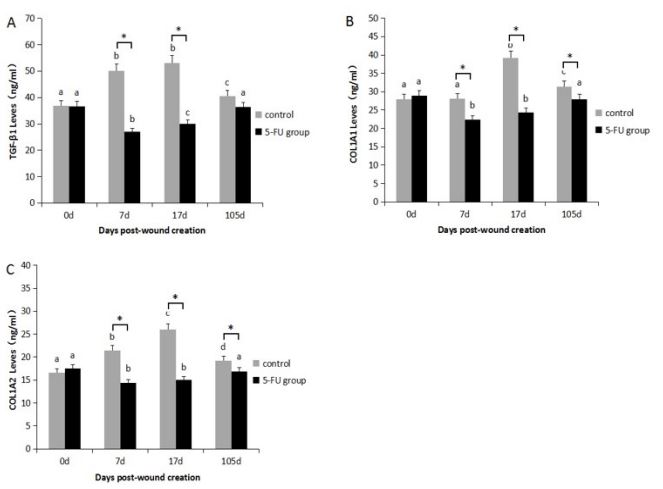
Collagen synthesis-related protein expression
Compared with the control group, TGF-β1, COL1A1, and COL1A2 protein expression was significantly reduced in the 5-FU
group in the proliferation stage of skin wound healing at days 7
and 17 (P < 0.05) (Figure 5).
Discussion
In this study, we examined the effect of 5-FU on wound healing
and HS formation in donkey skin model, and we specially investigated differences in proliferation stage. Our results showed that
5-FU could reduce collagen deposition and HS formation by inhibiting the proliferation of fibroblasts. It has been known that
scar formation is due to the proliferation of collagen fibers, and
disruption of the tissue structure [19-21]. 5-FU Could Inhibit 2
type I collagen gene (COL1A2) expression in human fibroblasts
[13]. Although donkey skin can secrete a lot of collagens, our
study suggests that 5-FU not only prolonged the period of proliferation during wound healing, but also reduced collagen deposition, allowing sufficient time for orderly arrangement of collagen
and scar-free healing. In the pre experiment, observation of skin
wound contraction combined with analysis of the expression of
collagen synthesis-related genes indicated that the proliferation
period of donkey skin wound healing occurred approximately
5-20 days after the trauma. Therefore, we started 5-FU treatment
before the granulation phase.
The TGF-β/Smad pathway is considered to be one of the main
mechanisms of fibroblast proliferation and is associated with regulation of the genes that cause scar fibrosis [22,23]. TGF-β1 promotes fibroblast growth, and increases the synthesis of collagen
and other extracellular matrixes to heal wounds [24-26]. 5-FU has
been implicated as an effective treatment for keloids formation by
inhibiting the expression of TGF-β1 gene and blocking the signaling
pathways involved in collagen synthesis [27,28]. Smad3 is activated by TGF-β1 to promote the expression of type I collagen genes
[29]. Fibroblast proliferation and collagen deposition is induced in
response to skin trauma, and can lead to HS formation. Inhibition
of Smad3 or increased expression of Smad7 results in reduced collagen deposition [30]. To investigate the role of the TGF-β/Smad
signaling pathway donkey skin wound healing and the effects of
5- FU treatment, we analyzed the expression of TGF-β, Smad3,
and Smad7. We found that the expression of TGF-β1, COL1A1 and
COL1A2 were significantly reduced in the 5-FU group at both the
gene and protein levels compared with the control group, which
may be mediated through TGF-β/Smad signaling pathway, indicating that 5-FU not only inhibited the growth of fibroblasts, but
also downregulated the expression of collagen.
Conclusion
This study shows that 5-FU reduces collagen deposition and
HS formation in donkey skin wounds, which highlights this as a
potential strategy to achieve scar-free wound healing.
Declarations
Funding: This study was supported by grant from National Natural Science Foundation of China (No. 31760643), Innovation and
development project of Shihezi University (No. CXFZ202007).
Ethics approval: All animal experiments in this study were approved by the Animal Ethics Committee at Shihezi University (Xinjiang, China).
Disclosure statement: No potential conflict of interest was reported by the author(s).
References
- Schilling JA. Wound healing. Surg. Clin. N. Am. 1976; 56: 859-874.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008; 453: 314-321.
- Volk SW, Wang Y, Mauldin EA, et al. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011; 194: 25-37.
- Trace AP, Enos CW, Mantel A, et al. Keloids and Hypertrophic Scars: A Spectrum of Clinical Challenges. Am J Clin Dermatol. 2016; 17: 201-223.
- Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol Med. 2010; 17: 113-125.
- Ma D, Chen L, Shi J, et al. Pigment epithelium-derived factor attenuates angiogenesis and collagen deposition in hypertrophic scars. Wound Repair Regen. 2020; 28: 684-695.
- Simon F, Bergeron D, Larochelle S, et al. Enhanced secretion of TIMP-1 by human hypertrophic scar keratinocytes could contribute to fibrosis. Burns. 2012; 38: 421-427.
- Liu XJ, Xu MJ, Fan ST, et al. Xiamenmycin attenuates hypertrophic scars by suppressing local inflammation and the effects of mechanical stress. J Invest Dermatol. 2013; 133: 1351-1360.
- Yang YW, Zhang CN, Cao YJ, et al. Bidirectional regulation of i-type lysozyme on cutaneous wound healing. Biomed Pharmacother. 2020; 131: 110700.
- Chin GS, Liu W, Peled Z, et al. Differential expression of transforming growth factor-beta receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001; 108: 423-429.
- Yin L, Zhao X, Ji S, et al. The use of gene activated matrix to mediate effective SMAD2 gene silencing against hypertrophic scar. Biomaterials. 2014; 35: 2488-2498.
- Huang D, Shen KH, Wang HG. Pressure therapy upregulates matrix metalloproteinase expression and downregulates collagen expression in hypertrophic scar tissue. Chin Med J. 2013; 126: 3321-3324.
- Wendling J, Marchand A, Mauviel A, et al. 5-fluorouracil blocks transforming growth factor-beta-induced alpha 2 type I collagen gene (COL1A2) expression in human fibroblasts via c-Jun NH2-terminal kinase/activator protein-1 activation. Mol Pharmacol. 2003; 64: 707-713.
- Peng WANG, Jun-sheng GONG, Jin-fu LI, et al. The research of changes of the Caspase-3 after 5-FU act on keloid fibroblasts. Chin J Med. 2016; 25: 60-64.
- Camacho LH, Garcia S, Panchal AM, et al. Exploratory study of hepatic arterial infusion oxaliplatin with systemic 5-fluorouracil/bevacizumab in patients with refractory solid tumor and extensive liver metastases. Clin Colorectal Cancer. 2010; 9: 311-314.
- Lv XG, Ji MY, Dong WG, et al. EBP50 gene transfection promotes 5-fluorouracil-induced apoptosis in gastric cancer cells through Bax- and Bcl-2-triggered mitochondrial pathways. Mol Med Rep. 2012; 5: 1220-1226.
- Simsek T, Firat P, Citirik M, et al. Short-term effects of subconjunctival injections of 5-fluorouracil on conjunctival epithelium. Cornea. 2010; 29: 727-731.
- Hong SJ, Wu WC , Lai YH , et al. Mechanism of 5-Fluorouracil-Induced Apoptosis on Cultured Corneal Endothelial Cells. Open J Apoptosis. 2014; 3: 5-15.
- Gong YY, Li XJ, Wang ML, et al. Collagen secretion from fibroblasts and the interventional effect of 5-fluorouracil in a time-dependent manner. J. Clin. Rehabil. Tissue Eng. Res. 2007; 11: 1060-1062, 1066.
- Grieb G, Steffens G, Pallua N, et al. Circulating fibrocytes--biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol. 2011; 291: 1-19.
- Wells A, Nuschke A, Yates CC. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol. 2016; 49: 25-36.
- Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001; 276: 17058-17062.
- Qin Z, Xia W, Fisher GJ, Voorhees JJ, Quan T. YAP/TAZ regulates TGF-β/Smad3 signaling by induction of Smad7 via AP-1 in human skin dermal fibroblasts. Cell Commun Signal. 2018; 16: 18.
- Gabriel V. Hypertrophic scar. Phys Med Rehabil Clin N Am. 2011; 22: 301-310.
- Liu RM, Desai LP. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015; 6: 565-577.
- Zhai XX, Tang ZM, Ding JC, Lu XL. Expression of TGF-β1/mTOR signaling pathway in pathological scar fibroblasts. Mol Med Rep. 2017; 15: 3467-3472.
- Çalıskan E, Gamsızkan M, Açıkgöz G, et al. Intralesional treatments for hypertrophic scars: Comparison among corticosteroid, 5-fluorouracil and botulinum toxin in rabbit ear hypertrophic scar model. Eur Rev Med Pharmacol Sci. 2016; 20: 1603-1608.
- Hussain A, Haque MW, Singh SK, et al. Optimized permeation enhancer for topical delivery of 5-fluorouracil-loaded elastic liposome using Design Expert: Part II. Drug Deliv. 2016; 23: 1242-1253.
- Bo L. Effects of SiRNA-mediated Smad3 gene silence on the expression of fibronectin and collagen in human keloid fibroblastsSiRNASmad3. J. Chongqing Med. Univ. 2010; 35: 522-526.
- Monteleone G, Kumberova A, Croft NM, et al. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001; 108: 601-609.