Introduction
While we typically think of cancer-related cognitive impairments as being associated with chemotherapy, research has suggested that cancer by itself may impact cognitive function. Studies
have demonstrated that 20-30% of patients with breast cancer
have impaired cognition prior to receipt of adjuvant chemotherapy or endocrine therapy [1]. Edwards et al, reported an elevated
prevalence of neurocognitive deficits –mild cognitive impairment
and dementia- in older adults with solid tumors and hematologic
malignancies when compared to population-based studies [2].
Some studies have shown evidence that cancer related cognitive impairment may be present before the initiation of treatment and that the cognitive profile found in assessment may
vary by type and localization of the cancer [3]. Different cognitive domains have been reported to be affected between breast,
colorectal, testicular, head and neck and hematological cancers
[4-8]. These changes may be due to, on one hand, to inconsistency in how neuropsychological tests are selected, performed,
and scored, but on the other hand, due to the different biology of
different cancers [9].
General consensus-based guidelines recommend that providers use cognitive assessment tools when evaluating older
patients with cancer [10]. Outside of the clinical trial setting,
incorporating a full neuropsychological evaluation into routine
oncology practice is not practically feasible. However, if a brief
evaluation with a short screening tool suggests possible cognitive
impairment, referral for a more comprehensive cognitive assessment may be warranted [11].
The “Clock Drawing Test (CDT)” has been proposed as an acceptable and time-efficient cognitive screening instrument, its reliability and validity has been extensively reported [12-14], and it
is validated in older cancer patients [15]. The person is instructed
to draw a clock with all the numbers and the hands pointing to a
specific time. Cognitive skills necessary for completion of the CDT
include: comprehension, planning, visual memory and reconstruction in a graphic image, visuospatial abilities, motor programming and execution, numerical knowledge, abstract thinking, auditory comprehension, verbal working memory, inhibition of the
tendency to be pulled by perceptual features of the stimulus and
concentration and frustration tolerance [16]. Both quantitative
(reports of the number of errors in the drawing) and qualitative
(reports of the type of errors in the drawing) scoring approaches
have been described and both have been associated with neuroa-natomical correlates [17,18]. The fact that clock drawing requires
a wide range of cognitive skills suggests that detailed qualitative
analyses of clock drawings could reveal the changes or disturbances of those cognitive skills, and neuropsychological profiles
can be developed [19]. This has been done for patients with
breast cancer in a descriptive way, but no particular follow up or
outcomes were assessed [20].
The aims of this study were to analyze qualitatively and quantitatively the CDT of older adults with different types of tumors,
as well as to analyze whether the CDT score or the types of errors
are associated with the type of tumor, history of previous chemotherapy, treatment modification and unplanned hospitalization.
Materials and methods
As part of usual care in the Senior Adult Oncology Program at
Moffitt Cancer Center (Florida, U.S.A.) a CDT is performed by the
nursing personnel at the first visit, as part of a geriatric assessment questionnaire called SAOP-3. We conducted a retrospective
analysis of those tests. Electronic medical records were reviewed
for first visits from January 2012 to December 2018. Patients over
65 years were eligible. Patients with brain metastasis, dementia
or mild cognitive impairment were excluded. The analyses of the
CDT were done as described by Parsey and Schmitter [21] by one of
the authors and a subset of CDT was rated by a second author for
interrater validity, with a moderate intraclass correlation for continuous CDT score (r=0.697, CI 0.504-0.824) and a moderate agreement for normal vs abnormal CDT (k=0.469) [22]. This method of
scoring gives a score from 1 to 3 to the errors made by the subject. Errors are divided in six categories: size of the clock, graphic
difficulties, stimulus-bound response, conceptual deficits, spatial
and/or planning deficits, and perseveration. Each category is subdivided in a particular type of error (see appendix). This method
of scoring has been used widely in the literature [18,21,23-30].
The CDT is scored as 16 – number of errors. Since the evaluation
at SAOP gives a pre drawn circle, we eliminated that category and
used 14 – number of errors, then the scores can be classified as
normal (14 or more points), mild impairment (12-13 points) and
cognitive impairment (11 or less points). The electronic medical
records were also reviewed to gather data about tumor characteristics, history of chemotherapy, treatment received, comorbidities, polypharmacy, alcohol and tobacco consumption, unplanned
hospitalizations and demographic variables reported to be associated with cognitive impairment (marital status, education level,
environmental exposure to toxics). Other data from the SAOP-3
was collected such as medications used by the patient and functionality on instrumental and basic activities of daily living.
Also, as part of an ongoing prospective practice improvement study, patients are reevaluated with a SAOP-3 screen every
3 months, which includes a CDT, so a follow up analysis of the
scores was performed in a subset of 48 patients who received this
3-months assessment to get insight in the evolution of the CDT
during treatment.
Correlations were tested for CDT score and comorbidities and
sample characteristics; Kruskal-Wallis tests were used to compare
the types of cancer and types of treatment with the CDT score.
One-way chi square tests were used to determine if a type of error was statistically more frequent in each type of cancer category. Fort the follow up group, a Wilcoxon test was performed to
compare the before and after treatment scores in the CDT. The
protocol was approved by the Advarra Institutional Review Board.
Results
Data from 364 individuals was used for analysis. Median age
was 77(70-97), 61.3% were female, mean score for CDT baseline was 12.27 (SD 1.54); 177 participants (48.6%) were completely independent in their basic activities of daily living (bathing,
dressing, transferences, continence, feeding), 272 participants
(74.7%) were completely independent in their instrumental activities of daily living (driving, preparing meals, shopping, managing finances, using a phone, taking medications). Types of cancer were grouped as follows: breast (n=165), gastrointestinal (GI; n=82), head and neck (H&N; n=22), genitourinary (GU; n=69),
and others (n=26). Table 1 presents the complete characteristics
of the sample. No correlation was found between comorbidities
(neither to individual nor to the number of comorbidities a single
patient had) and the CDT score. The individual comorbidities we
analyzed were depression, hypothyroidism, heart failure, chronic
kidney disease, type 2 diabetes mellitus, hypertension, chronic
obstructive pulmonary disease, ischemic heart disease, history of
stroke or transient ischemic attack, and hypoacusis. No correlation was found between de CDT score and the number of drugs
taken. A Pearson correlation was found to be statistically significant between age and the CDT score (p=<0.001). We found in
the electronic health records that before CDT, 16.8% and 23.4%
of the participants reported using benzodiazepines and opioids
respectively, and since those drugs have been classically reported to cause cognitive alterations, Mann-Whitney U Tests were
performed to compare the CDT score and the use of these drugs
without statistically significant results. Table 2 has the CDT scores
of the sample by type of cancer, while Table 3 has the scores of
the CDT by exposure to chemotherapy and metastasis.
To compare the effect of the type of cancer on the CDT score,
Kruskal-Wallis test was conducted. The result was significant [X2
(4)=19.397, p=0.001]. Post hoc comparisons were made to find
where the differences between groups were located. Statistically
significantly differences were found between Breast and GI (p=0.030, effect size 0.019); Breast and H&N
(p<0.001, effect size 0.074); Breast and GU (p=0.007, effect size
0.031); GI and H&N (p=0.013, effect size 0.060); H&N and others
(p=0.016, effect size 0.12).
In the group of patients that had a 3 month follow up CDT
(N=48), Kruskal-Wallis was performed to see if the type of treatment received had any effect on the CDT score. No statistically
significantly differences were found. No patient in the “other tumor” category had a follow-up CDT. A signed ranks Wilcoxon test
was performed to compare before and after treatment scores in
the CDT. No statistically significantly difference was found (Negative ranks=14, positive ranks=19, ties=15; p=0723).
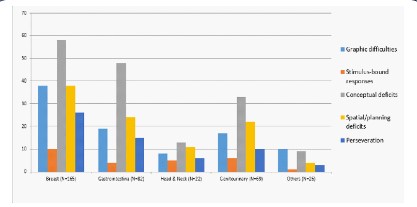
One-way chi square tests were performed to determine
whether a pattern exists in the type or errors the subjects made
in the CDT. In the breast cancer group, errors in the conceptual
deficit category (58) were statistically more frequent than the
other error categories, X2 (4, N=170) = 36.706, p=<0.001. While
errors in misinterpretation of time (54) were the most frequent
subtype of error. (X2 (11, N=179) = 171.413, p=<0.001). In the GI
cancer group, errors in the conceptual deficit category (48) were
statistically more frequent than the other error categories, X2 (4,
N=110) = 48.273, p=<0.001. Errors in misinterpretation of time
(44) were the most frequent subtype of error (X2 (11, N=118) =
191.89, p=<0.001). In the GU cancer group, errors in the conceptual deficit category (33) were more frequent than the other error
categories (X2 (4, N=88) = 25.523, p=<0.001). Errors in misinterpretation of time (28) were the most frequent subtype of error (X2
(11, N=113) = 63.602, p=<0.001).
In the head and neck cancer group, errors in the conceptual
deficit category (13) were the most common, but not statistically
significantly difference was found (X2 (4, N=43) = 5.256, p=0.262);
errors in misinterpretation of time (10) were the most frequent subtype of error (X2 (12, N=53) = 23.283, p=0.025)
In the other cancer group (N=23), errors in the graphic difficulties category (10) were more frequent than the other error categories (X2 (4, N=27) = 11.333, p=0.023). No subtype of error was
statistically more frequent (X2 (10, N=32) = 18, p=0.055).
The most common category of error in the CDT for breast, GI,
GU and H&N was conceptual deficits. While perseveration was
the most common in the “others” group. Misinterpretation of
time was the subtype of error the most common in all the population.
It is interesting that 3 subjects had neglect of the left hemispace, all were female, had gastrointestinal tumors, were taking
more than 4 drugs at the time of evaluation, had 12 or more years
of education; two of the 3 needed reductions in the chemotherapy dosage, and all of them had impairment in at least one of the
activities of daily living interrogated in the SAOP-3 questionnaire.
There were no common comorbidities between these 3 participants. Also, 2 subjects had numbers written counterclockwise,
but no common variables were found among them. These 2 types
of error have not been described as common in non-cognitively
impaired populations. Figure 1 has the subtype of errors according to cancer type.
Mann-Whitney U tests were performed to compare the baseline CDT of patients that had chemotherapy 3 years or more
before baseline (n=69), patients that had chemotherapy in the 3
years before baseline (n=179), and patients that had metastatic
disease at baseline (n=197), with patients that did not have any of
these conditions. None of the tests were statistically significant.
(p=0.751, p=0.179, p=0.417, respectively). In the three groups,
conceptual deficits and misinterpretation of time were the most
common errors. No correlation was found between baseline CDT
score and treatment modifications or non-planned hospitalizations.
Discussion/conclusion
The aim of this study was to describe the CDT of older cancer patients both quantitatively (the total numeric score of
the drawing) and qualitatively (the type of errors found in the
drawing) and to identify possible associations between the score
and types of errors with the type of tumor, and exposition to cancer treatment. The sample was composed of adults 65 years and
older that all had a CDT as usual care in their first visit to the SAOP
at Moffitt Cancer Center. The CDT was scored as described by Parsey and Schmitter [21].
Table 1: Baseline characteristics and demographics.
N=364 |
|
Age in years, median (range) |
77(70-97) |
Female (%) |
223(61.3) |
Has a partner (%) |
236(64.8) |
Currently employed (%) |
25(6.9) |
12 years or more of formaleducation (%) |
285(78.3) |
Number of drugs taken,median (range) |
8(0-27) |
Subjects taking 4 or more drugs, n (%) |
349(87.6) |
Independent in BADL, n (%) |
177(48.6) |
Dependent in one BADL, n (%) |
137(37.6) |
Dependent in two BADL, n (%) |
43(11.8) |
Dependent in three or more BADL, n (%) |
7(1.9) |
Independent in IADL, n(%) |
272(74.7) |
Dependent in one IADL, n(%) |
49(13.5) |
Dependent in two IADL, n(%) |
11(3) |
Dependent in three or more IADL, n(%) |
32(8.8) |
Type of cancer, n(%) |
|
Breast |
165(45.3) |
Gastrointestinal |
82(22.5) |
Head and Neck |
22(6) |
Genitourinary |
69(19) |
Others |
26(7.1) |
ECOG*, n(%) |
|
0 |
156(42.9) |
1 |
155(42.6) |
2 |
45(12.4) |
3 |
5(1.4) |
Metastasis at baseline evaluation, n(%) |
197(54.1) |
Treatment intention, n(%) |
|
No treatment |
21(5.8) |
Neoadjuvant |
13(3.6) |
Adjuvant |
104(28.6) |
Palliative |
203(55.8) |
Curative |
23(6.3) |
Had previous cancer, n(%) |
105(28.8) |
Had chemotherapy in the 3 previous years, n(%) |
179(49.2) |
CDT normal (%) |
126(34.6) |
CDT mean score (SD) |
12.59(1.46) |
*Information was missing for 3 subjects.
BADL: Basic activities of daily living (bathing, dressing, transferences,
continence, feeding), IADL: Instrumental activities of daily living (driving,
preparing meals, shopping, managing finances, using a phone, taking
medications); ECOG: Eastern Cooperative Oncology Group performance
status; CDT: Clock drawing test, SD: Standard deviation
Table 2: CDT scores according to type of cancer.
|
Breast(n=165) |
GI(n=82) |
H&N(n=22) |
GU(n=69) |
Others(n=26) |
| Mean |
12.88 |
12.52 |
11.5 |
12.26 |
12.65 |
| Normal (14 points) |
64(38.8%) |
25(30.5%) |
3(13.6%) |
21(30.4%) |
11(42.3%) |
| Mild impairment (12-13) |
78(47.3%) |
42(51.2%) |
8(36.3%) |
25(36.2%) |
10(38.5%) |
| Cognitive impairment (≤11) |
23(13.9%) |
15(18.3%) |
11(50%) |
23(33.3%) |
5(19.2%) |
GI: Gastrointestinal; H&N: Head and neck; GU: Genitourinary
Table 3: Clock drawing test scores according to previous treatment and metastasis.
|
Chemotherapy older than 3 years (n=69) |
Chemotherapy in previous 3 years (n=179) |
No history of chemo (n=197) |
Metastasis at baseline(n=151) |
| Mean |
12.58 |
12.73 |
12.54 |
12.42 |
| Normal (14points) |
20(29%) |
64(35.8%) |
62(31.5%) |
47(31.1%) |
| Mild impairment (12-13) |
37(53.6%) |
85(47.5%) |
91(46.2%) |
64(42.4%) |
| Cognitive impairment (≤11) |
12(17.4%) |
30(16.8%) |
44(22.3%) |
40(26.5%) |
Across all types of cancers, the most common error was in the
conceptual deficits category. This finding was also reported by
Spenciere et al. [30]. That study was performed with a sample of
49 community dwelling older adults over 60 years from Brazil. Interestingly, in that healthy older population, all drawings had spatial and/or planning deficits and the mean score was 11.4 which is
lower than in our sample. This can be explained by the higher education level reported for developed countries or by the fact that
there was a high prevalence of depression in the Spenciere study, which in our sample was only present in 15.7% of the subjects. In
this healthy older adult population, no patients had severe graphic difficulties nor neglect of the left hemispace. These errors
were found in our sample, and they could signify that the subject that presents them does have cognitive impairment, whether
this is caused by the cancer itself, the treatment, or other patient
characteristics can´t be known with our data, a prospective study
could clarify this. In total, 34 subjects of our sample had one of
these types of errors (29 had numbers written outside the clock, 3 had neglect of left hemispace and 2 had the numbers written
counter clockwise). The small number of patients with these errors in our sample makes it impossible to extrapolate or doing
a deeper analysis but nevertheless these should be considered
for further studies, especially because several patients needed a
modification of treatment or had unplanned hospitalizations, and
it highlights the importance of sending these patients for further
cognitive examination.
Making this more interesting, a study by Teixeira et al. [31],
also found that severe graphic difficulties, neglect of the left
hemispace, numbers written outside of the clock face or numbers written counterclockwise were not found in healthy older
patients. They found that in cognitively impaired individuals the
most common error were conceptual deficits, followed by planning mistakes, size of the clock, perseveration and stimulus bound
response. In this study they classified the patients by years of formal education (1-4 years, 5-8 years, and >8 years) and found that
the misrepresentation of the clock and counterclockwise number
display occurred only in the least educated group, mild graphic
difficulties occurred in all groups, and that neglect of the left hemi-space of the clock was not observed in any level of schooling.
In our study the most common conceptual deficit was the hands
being the same length. In the aforementioned studies, this was
found as commonly in the healthy population. Also, this error was
associated with reduced cerebral blood flow in the posterior and
middle temporal lobes in studies with single photon emission CT
[29,32,33]. Since atrophy in these regions has been described as
normal in aging, this kind of conceptual deficit could be just a
marker of aging. Further research is needed to confirm this.
Another interesting finding in our study is that the mean score
in the CDT was not significantly changed at baseline and follow
up, even after receiving some type of cancer treatment (chemotherapy, hormonal or targeted). In a study by Hurria and colleagues, 39% of patients had a decline in cognitive function from
baseline, 50% had no change, and 11% had improvement in cognitive testing [34]. They performed a complete cognitive evaluation, while we only used a screening test, which reinforces the
importance of complete cognitive examination in those that have
positive screening. This can be reflective of the insensitivity of the
CDT to detect subtle changes over a short period of time between
examinations. We must acknowledge that our follow-up sample
of 48 patients has a low power of detecting subtle changes, so a
prospective study should be performed.
We had a high heterogeneity of cancer subtypes and treatment regimens, and a small sample, which makes it hard to extrapolate our findings. Even while the SAOP-3 does a pretty good baseline evaluation of our patients, the retrospective nature of our
study could have missed some important clinical data, as it might
have been lost or unrecorded. On the other hand, we added to
the evidence that some types of errors in the CDT (neglect of left
hemi-space, numbers written counterclockwise) that suggest the
need for a deeper cognitive evaluation even if the overall (quantitative) score classifies the patient as normal, are present among
older cancer patients even before starting treatment. Adequate
working memory has been associated with decision-making ability [35], which is of paramount importance when dealing with a
cancer diagnosis and treatment possibilities, as well as when navigating medical services. Thus, the detection of those individuals with cognitive problems and their timely referral for a complete
evaluation could be carried out with the CDT, both to find modifiable causes (polypharmacy, vitamin deficiencies, lack of control
of comorbidities) and to appoint substitutes and representatives
in decision-making.
This study was made with a retrospective convenience sample,
and our sample size of participants with a follow up CDT provided
a low power in assessing the effect of treatment. A prospective
study with a higher number of subjects in each cancer group and
a healthy group for comparison, could help clarify if the errors in
the CDT are related to specific pathophysiological effects of the
type of cancer, effects of a particular treatment, or characteristics
of aging.
In this study, across different types of cancers, the most common error in the CDT was in the conceptual deficits category, with
misinterpretation of time being the most frequent subtype of error. No correlation was found between comorbidities, previous
exposure to chemotherapy or history of a previous cancer and
the CDT score. Breast cancer patients had significantly different
CDT scores compared to the other groups. At 3 months follow
up, there were no associations between the type of treatment
received and the CDT score.
References
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment.
Int Rev Psychiatry. 2014; 26(1): 102-13.
- Edwards BJ, Zhang X, Sun M, Holmes HM, Ketonen L, Guha N, et
al. Neurocognitive deficits in older patients with cancer. J Geriatr
Oncol. 2018; 9(5): 482-7.
- Mayo SJ, Lustberg M, HMD, Nakamura ZM, Allen DH, Von Ah D, et
al. Cancer-related cognitive impairment in patients with non-central nervous system malignancies: an overview for oncology providers from the MASCC Neurological Complications Study Group.
Support Care Cancer. 2021; 29(6): 2821-40.
- Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective
longitudinal study of chemotherapy-induced cognitive changes in
breast cancer patients. Support Care Cancer. 2011; 19(10): 1647-56.
- Vardy JL, Dhillon HM, Pond GR, Rourke SB, Bekele T, Renton C, et
al. Cognitive Function in Patients With Colorectal Cancer Who Do
and Do Not Receive Chemotherapy: A Prospective, Longitudinal,
Controlled Study. J Clin Oncol. 2015; 33(34): 4085-92.
- Wefel JS, Vidrine DJ, Veramonti TL, Meyers CA, Marani SK, Hoekstra HJ, et al. Cognitive impairment in men with testicular cancer
prior to adjuvant therapy. Cancer. 2011; 117(1): 190-6.
- Piai V, Prins JB, Verdonck-de Leeuw IM, Leemans CR, Terhaard CHJ,
Langendijk JA, et al. Assessment of Neurocognitive Impairment
and Speech Functioning Before Head and Neck Cancer Treatment.
JAMA Otolaryngol Head Neck Surg. 2019; 145(3): 251-7.
- Hshieh TT, Jung WF, Grande LJ, Chen J, Stone RM, Soiffer RJ, et al.
Prevalence of Cognitive Impairment and Association With Survival
Among Older Patients With Hematologic Cancers. JAMA Oncol.
2018; 4(5): 686-93.
- Horowitz TS, Treviño M, Gooch IM, Duffy KA. Understanding the
Profile of Cancer-Related Cognitive Impairments: A Critique of
Meta-Analyses. J Natl Cancer Inst. 2019; 111(10): 1009-15.
- Hurria A, Wildes T, Blair SL, Browner IS, Cohen HJ, Deshazo M, et al.
Senior adult oncology, version 2.2014: clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 2014; 12(1): 82-126.
- Magnuson A, Mohile S, Janelsins M. Cognition and Cognitive Impairment in Older Adults with Cancer. Curr Geriatr Rep. 2016; 5(3):
213-9.
- Juby A, Tench S, Baker V. The value of clock drawing in identifying
executive cognitive dysfunction in people with a normal Mini-Mental State Examination score. Cmaj. 2002; 167(8): 85964.
- Kirby M, Denihan A, Bruce I, Coakley D, Lawlor BA. The clock
drawing test in primary care: sensitivity in dementia detection and
specificity against normal and depressed elderly. Int J Geriatr Psychiatry. 2001; 16(10): 935-40.
- Ketelaars L, Pottel L, Lycke M, Goethals L, Ghekiere V, Santy L, et
al. Use of the Freund clock drawing test within the Mini-Cog as a
screening tool for cognitive impairment in elderly patients with or
without cancer. J Geriatr Oncol. 2013; 4(2): 174-82.
- Lycke M, Ketelaars L, Boterberg T, Pottel L, Pottel H, Vergauwe P, et
al. Validation of the Freund Clock Drawing Test as a screening tool
to detect cognitive dysfunction in elderly cancer patients undergoing comprehensive geriatric assessment. Psychooncology. 2014;
23(10): 1172-7.
- Shulman KI. Clock-drawing: is it the ideal cognitive screening test?
Int J Geriatr Psychiatry. 2000; 15(6): 548-61.
- Tranel D, Rudrauf D, Vianna EP, Damasio H. Does the Clock Drawing
Test have focal neuroanatomical correlates? Neuropsychology.
2008; 22(5): 553-62.
- Eknoyan D, Hurley RA, Taber KH. The clock drawing task: common
errors and functional neuroanatomy. J Neuropsychiatry Clin Neurosci. 2012; 24(3): 260-5.
- Spenciere B, Alves H, Charchat-Fichman H. Scoring systems for the
Clock Drawing Test: A historical review. Dement Neuropsychol.
2017; 11(1): 6-14.
- Overcash J, Perry M. Cognitive Screening: Using the Clock-Drawing
Test to Assess for Preexisting Deficits in Older Women Diagnosed
With Breast Cancer. Clin J Oncol Nurs. 2017; 21(4): 489-98.
- Parsey CM, Schmitter-Edgecombe M. Quantitative and qualitative
analyses of the clock drawing test in mild cognitive impairment
and Alzheimer disease: evaluation of a modified scoring system. J
Geriatr Psychiatry Neurol. 2011; 24(2): 108-18.
- Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass
Correlation Coefficients for Reliability Research. J Chiropr Med.
2016; 15(2): 155-63.
- Aguilar-Navarro SG, J M-AA, A. S-CM, J. H-CF, A. G-GL, Fátima
R-G. Validation of the Clock Drawing Test Scoring Method in older
adults with neurocognitive disorder.: Salud Men; 2018. p. 17986.
-
Shao K, Dong F-M, Guo S-Z, Wang W, Zhao Z-M, Yang Y-M, et al.
Clock-drawing test: Normative data of three quantitative scoring
methods for Chinese-speaking adults in Shijiazhuang City and clinical utility in patients with acute ischemic stroke. Brain and behavior. 2020; 10: e01806.
- Hemmy L, Linskens E, Silverman P, Miller M, Talley K, Taylor B, et
al. Brief Cognitive Tests for Distinguishing Clinical Alzheimer-Type
Dementia From Mild Cognitive Impairment or Normal Cognition in
Older Adults With Suspected Cognitive Impairment: A Systematic
Review. Annals of internal medicine. 2020; 172.
- Abawi M, Vries R, Stella P, Agostoni P, Boelens D, Jaarsveld R, et
al. Evaluation of Cognitive Function Following Transcatheter Aortic
Valve Replacement. Heart, Lung and Circulation. 2018; 27: 1454–61.
- Turcotte V, Gagnon M-E, Joubert S, Rouleau I, Gagnon J-F, Escudier
F, et al. Normative data for the Clock Drawing Test for French-Quebec mid- and older aged healthy adults. The Clinical Neuropsychologist. 2018; 32.
- Duro D, Tábuas-Pereira M, Freitas S, Santiago B, Botelho M, Santana I. Validity and Clinical Utility of Different Clock Drawing Test
Scoring Systems in Multiple Forms of Dementia. Journal of Geriatric Psychiatry and Neurology. 2018; 31: 089198871877443.
- Duro D, Cerveira P, Santiago B, Cunha MJ, Pedroso de Lima JM,
Botelho MA, et al. Clock drawing test in mild cognitive impairment:
Correlation with cerebral perfusion in single-photon emission
computed tomography. Neuropsychology. 2019; 33(5): 617-32.
- Spenciere B, Mendes-Santos LC, Borges-Lima C, Charchat-Fichman
H. Qualitative analysis and identification of pattern of errors in
Clock Drawing Tests of community-dwelling older adults. Dement
Neuropsychol. 2018; 12(2): 181-8.
- Teixeira Fabricio A, Aprahamian I, Sanches Yassuda M. Qualitative
analysis of the Clock Drawing Test by educational level and cognitive profile. Arq Neuropsiquiatr. 2014; 72(4): 289-95. 32.
- Ueda H, Kitabayashi Y, Narumoto J, Nakamura K, Kita H, Kishikawa
Y, et al. Relationship between clock drawing test performance and
regional cerebral blood flow in Alzheimer’s disease: a single photon emission computed tomography study. Psychiatry Clin Neurosci. 2002; 56(1): 25-9.
- Matsuoka T, Narumoto J, Okamura A, Taniguchi S, Kato Y, Shibata
K, et al. Neural correlates of the components of the clock drawing
test. Int Psychogeriatr. 2013; 25(8): 1317-23.
- Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, Lachs MS,
et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc. 2006; 54(6): 925-31.
- Lindquist LA, Miller-Winder AP, Schierer A, Murawski A, Opsasnick
L, Curtis LM, et al. Aspects of cognition that impact aging-in-place
and long-term care planning. J Am Geriatr Soc. 2022; 70(9): 2646-52.