Introduction
Nipple discharge or breast discharge, as one of the most general complaints incurred by breast-related diseases different from
other symptoms such as breast pain and breast lumps, presents
particularly common in women patients aged 30-50 with an incidence of 4.8%-7.4% [1,2]. The dominating etiology of incurring
Pathologic Nipple Discharge (PND) is a benign papilloma, with an
incidence of 52%-57% [3-5]. Duct ectasia is another of the most
common benign causes of PND, representing approximately 14%-
33% of the cases [3], while breast cancers such as Ductal Carcinoma in Situ (DCIS) take up 5%-15% of cases of pathologic nipple
discharge. In addition, breast infections, including periductal mastitis and breast abscess, are also incurring etiologies [6-9]. Due
to its possible relevance for breast cancer, PND has posed significant attention regarding its significance of excluding deterioration if immediate diagnostic modalities and operative treatment are
performed accurately and efficiently. Nowadays, the techniques
for diagnosing the incurrence of PND focus on mammography,
routine sonographic examination and Contrast-Enhanced Ultrasound (CEUS), mammary fiberoptic ductoscopy, and Magnetic
Resonance Imaging (MRI) to find the most efficient and accurate
diagnostic strategy for a better decision [10]. These methods are
also enhanced by the novel technique device Vacuum-Assisted
Breast Biopsy (VABB) guided by ultrasound as a minimally invasive
approach in breast puncture for evaluating the qualitative diagnosis and effective treatment. Given the cost efficiency and state-ofart techniques availability of Sono Vue Contrast-Enhanced Ultrasound (CEUS)-guided VABB, this project review has summarized
the illustrative terminologies and diagnostic imaging techniques
used in these methods.
Indications and classifications of nipple discharge
What is nipple discharge, and what has caused it?
Nipple discharge is generally classified as breast discharge
in normal conditions like physiological discharge and abnormal
conditions such as galactorrhea and pathologic nipple discharge
founded on their exhibiting characteristics and causes of presentation [2].
Physiological/benign discharge
The benign occurrence of discharge refers to physiological
lactation commonly, including milk and colostrum production,
or sometimes with external stress incurring some drops of sticky or dark-colored viscous fluid that are normal for the process
of mammogenesis or lactogenesis during the puerperal period.
The physiological discharge is responsive to a series of hormonal
regulations and external physical or biochemical stimuli-induced
hormonal secretion [2,10].
Nonpuerperal galactorrhea/abnormal discharge
Galactorrhea is not an indication of primary breast pathology
compared to pathological nipple discharge. However, it refers to
nonpathological nipple discharge during the nonpuerperal period,
generally after more than 1 year of breastfeeding. Galactorrhea is
mainly manifested as bilateral milky secretion and, in a few cases,
as unilateral with light-colored discharge except when it contains
blood. This abnormal circumstance is associated with the improper escalation of prolactin release (hyperprolactinemia), which
could be secondary to medications of several pharmacological
categories, including hormones, psychotropics, and antihypertensives. It can also be caused by other pathological changes such as
pituitary adenoma, endocrine disorders such as hyperthyroidism,
hypothyroidism, and renal failure, as well as other breast stimulation or chest complications [2].
Pathological Nipple Discharge (PND)
Pathological Nipple Discharge (PND) is a breast-related disease’s most significant aberrant symptom. It is characterized by
sanguineous, blood-stained, or serous-like (transparent or colored) fluid secretory production rather than regular milk lactation with the occurrence unilaterally within a single duct orifice
of the nipple. Although the color of breast nipple secretory fluid
functions as the alarm bell for further clinical evaluation, it never
makes a decisive diagnosis to differentiate the benign from malignance, even if it indicates PND [2,10]. The common etiologies
with which it correlates are described below:
Intraductal Papilloma (IDP): The benign papillary tumor is the
most common cause of PND, especially for non-pregnant or nonpuerperal females with the apparent indications of presenting
sanguineous discharge without the palpable mass [2]. This lesion
is growing intraductal and adherent to the mammary duct wall localizing near the nipple orifice, occasionally concealing the malice
existence of atypia or Ductal Carcinoma in Situ (DCIS) [10]. According to diagnostic imaging studies for judging PND with routine
diagnostic investigation approaches, the papillomas’ detection
sensitivity in mammography was 62.9%, in sonography 72%, and
in ductoscopy 86.6% [11].
Duct ectasia: Mammary duct ectasia (MDE) is the second most common benign etiology causing PND, which can be present in
15% to 20% of the patients who have suffered from nipple discharge [10,12]. Women over 50 could be most affected during
the perimenopausal phase or postmenopausal period with indications of white, green, black, or grey-colored nipple discharge
unilaterally or bilaterally, and even breast pain or tenderness
[2]. This is a non-proliferative and non-invasive inflammatory disease characterized by focal dilatation of endoluminal lactiferous
ducts filled with keratin obstructions or thick clogged secretions,
as well as changes in duct wall elastin. This abnormal condition
consequently leads to chronic inflammation and periductal fibrosis [2,9,10]. MDE could overlap some clinical manifestations
in benign conditions such as periductal mastitis. Magnetic Resonance Imaging (MRI) also appears as a module of enhancement,
similar to DCIS presents [9]. Thus clinical imaging assessment or
even biopsy and histopathologic examination of the excised tissue
are required to decide the differential diagnosis.
Breast carcinoma: DCIS is a heterogeneous group of intraductal
tumors forming neoplastic lesions from the lining of breast mammary ducts and lobules. Sometimes DCIS occurs together with
PND [13]. However, it is the least likely incurring cause among the
three primary etiologies for PND, and particularly concomitant
with a palpable mass, PND may thus be regarded as an alarm sign
which is of significance in cancer detection [10].
Breast infection: Infectious diseases linked to PND mainly
concern breast periductal mastitis and abscess formation. The
clinical indications are multi-colored discharge, swelling, redness
or tenderness, and even fever in some cases. Imaging modalities,
e.g., sonography, can present the purulence as a hypoechoic lump
or multiloculated fluid with a rim of thickness and echogenicity.
The treatment focuses on empiric antibiotics, surgical drainage,
and abscess excision [14].
Diagnostic imaging modalities
What are the current diagnosis techniques? How do imagining studies help clinicians?
Initial clinical evaluation of a thorough history inquiry and proper physical examination is required in all female patients who
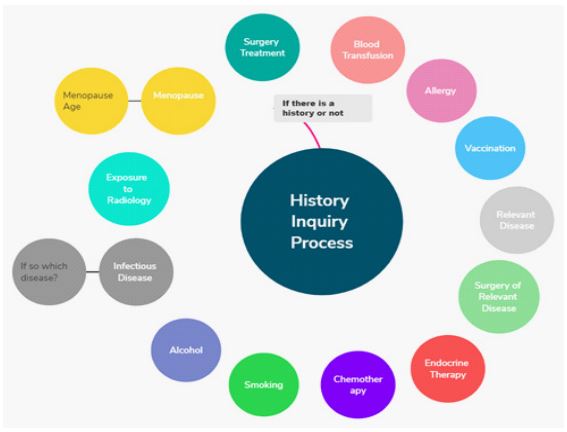
have suffered from non-lactational nipple discharge [2]. The history inquiry has contained several factors related to prior medical
conditions of patients with PND to define if there is a history or
not that could have an impact on current clinical manifestations
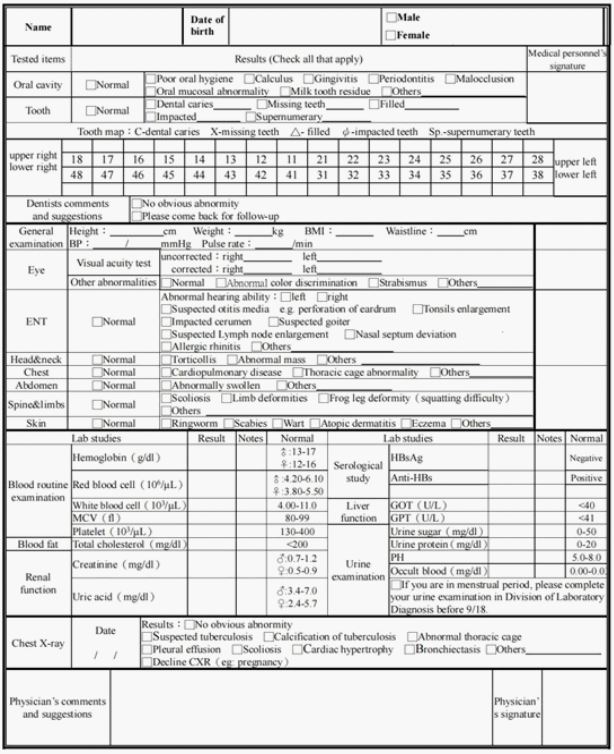
(Figure 1), while a physical examination is to examine the main
parts from head and neck, torso and four limbs including blood
routine examination to get a general realization for the general
conditions of patients in case of any other incurrences (Figure 2).
Apart from initial clinical evaluation, it starts from standard imaging diagnostic evaluation of conducting mammography to reveal
physiological discharge or pathological discharge. Afterward, patients with PND will need further up-to-date and most commonly
available imaging diagnostic investigation in a step-wise approach
of sonographic examination, ductoscopy, and MRI to determine
the explicit benign findings or malignancy suspect. For instance,
galactography/ductography, cytological smear, and biopsy are not
widely used during clinical diagnostic appraisal, particularly for
galactography. The latter methods will be conducted only for further suspicion of cancer probability of malignant conditions. Furthermore, clinical imaging findings for diagnosing patients with PND symptoms can vary depending on the potential etiology and
imaging strategic methods [15].
Mammography
Mammography, as the front-line clinical diagnostic imaging
modality for breast disorders usually followed by sonography/
ultrasound, is still practically recommended as a crucial instrument of starting-point investigation and diagnosis for patients
with PND to rule out the malignant possibility of breast lesions
given that it has the medium relative radiation level (01-1 mSv)
compared to other imaging modalities such as sonography and
MRI without radiological absorption [16]. The principle of it is to
conduct low-energy X-rays (30 kVp) of ionizing radiation to create
visualized images typically for detecting breast lumps or micro calcifications characteristically [17]. Following a meta-analysis
study of collecting 36 studies of 3764 patients who have accepted the mainstream imaging diagnosis in terms of detection of
malignity lesions, mammography has shown the highest average
specificity with 93% and lowest sensitivity with 22% as well as
the highest value in positive predictive value with 46% but diagnostic accuracy rate of medium ranking with 76% compared to
the highest value of ductoscopy with 88% [18] which is to some
extent qualified to identify the patients in PND without high-risk
lesions or carcinoma in breast indicating whether the subsequent
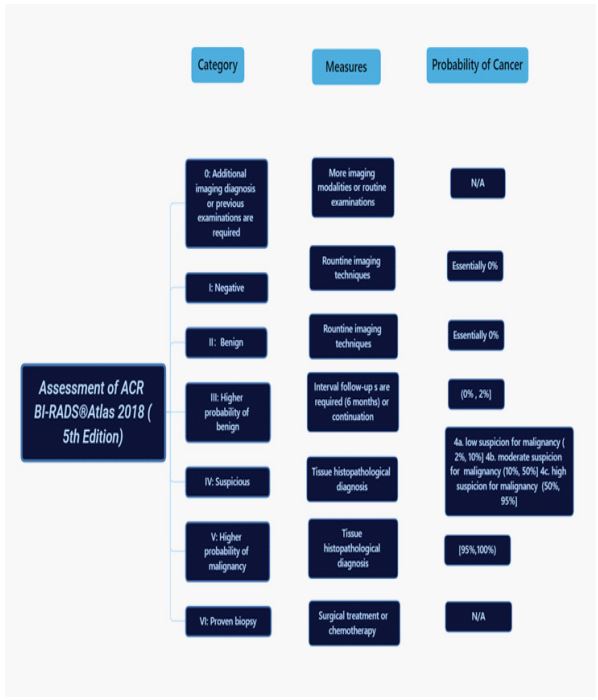
imaging studies are essentially required. Criteria (Figure 3), as a
dividing line between benign and malignity in breast lesions with
breast imaging reporting and interpretations from not only applying to mammography but also including ultrasound and MRI, is
referring to ACR BI-RADS® (American College of Radiology, Breast
Imaging-Reporting, and Data System) [19], which is a general risk
appraisal and quality control lexicon to be designed to standardize breast diagnostic studies: it has been categorized to seven assessment classifications while BI-RADS 0-III considered as benign
conditions. In contrast, BI-RADS IV-VI is considered suspicious malignant or proven malignancy [20]. However, findings of morphologic changes on mammography are prone to be detected better,
ranging from normality to retro areolar dilated ducts, structural
distortion and asymmetry, periductal micro calcifications, inverted nipple, and breast mass but not for cancer and comprehensively intraductal detection.
Sonographic examination
The routine sonographic examination is a widespread technique in clinical imaging practice in the field of breast imaging,
which is supplementary primarily to mammography, especially if
the result of mammography is negative for patients with PND [21] nd has earned wide recognition due to its characteristics of noninvasive and free of ionizing radiation and even rated and ranked
highest in clinical imaging scenarios with its features of criteria
according to Lee et al. review of ACR Appropriateness Criteria®
Evaluation of Nipple Discharge [16]. As for the malignity detection in patients suffering from PND, sonography presents a sensitivity of 50%, specificity of 69%, positive predictive value is 31%
and negative-positive value is 83% [18]; among other imaging
modalities, its performance is not as satisfactory as other specific features, but it is still recommended for diagnosis for some
benign conditions such as intraductal papilloma causing intraductal nodule and ductal ectasia which are the first two significant
etiologies causing PND [22]. It also takes advantage of detecting
palpable or non-palpable breast mass and the involved ducts. If a
mass is detected via physical examination or mammographically
identified, it would be better to investigate if it is solid, cystic, or
intraductal [15]. The routine ultrasound has the modes of chromatic doppler ultrasound with Color Doppler Flow Imaging (CDFI)
and Color Doppler Energy (CDE) to observe the blood flow and
distribution, while chromatic doppler could exhibit larger blood
vessels and higher blood flow velocity but not microvessels then
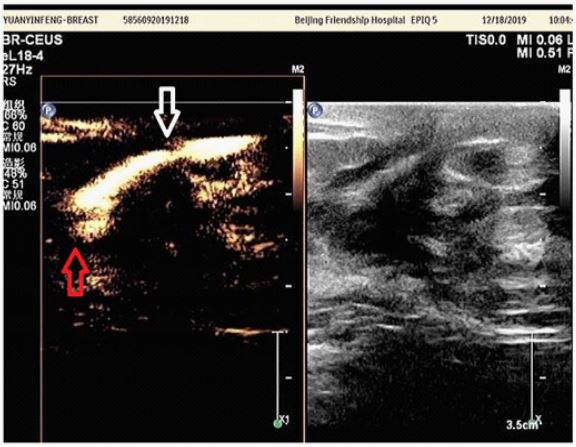
switch to Contrast-Enhanced Ultrasound (CEUS) probe and compression coupled with contrast agent (sulful hexafluoride microbubbles such as Sono Vue, diameter is tiner than erythrocytes)
administrated intravenously could show the perfusion of capillaries in tissues or lesions with a distribution of microbubbles in
microvessels with a better visualization of detected mass or even
benign or malignant breast tumors since angiogenesis is significant for cancer metastasis, growth and invasiveness correlated to
prognostic effect for which CEUS can provide better imaging and
evidence for diagnosis (Figure 4) [23]. CEUS is quite essential and
practical during necessary intraoperative procedures to guide minimally invasive microductectomy, namely the VABB technique.
Ductoscopy
Mammary ductoscopy is conventionally suggested and recommended for further investigation when mammogram and sonographic examination lacks the ability to diagnose the causative
lesion or make a diagnostic conclusion of patients with PND even along with the therapeutic effectiveness for the treatment by laser ablation or mechanical clearance of intraductal lesions such
as papillomas [15,24,25]. This invasive micro-endoscopic technique, free of ionizing radiation, is generally conducted with La Du
Scope-T flex as the fiberoptic scope with an outer diameter of 1.0
mm under local anesthesia using diluted lidocaine (0.5%) for the
povidone-iodine solution cleaned nipple-areola complex, from
which can provide a real-time visualization access to the endolumenal lactiferous duct of the breast via cannulation of nipple unilaterally [25,26]. Aiming to identify where the part or complete intraductal lesion locates resulting in obstructed duct or mammary
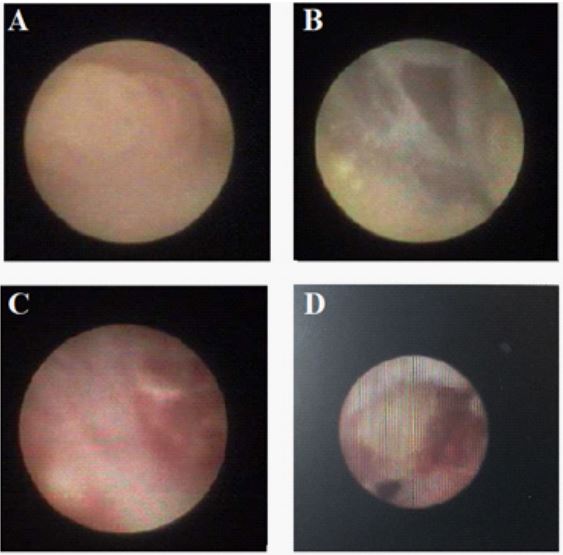
duct abnormalities (Figure 5), ductoscopy has been demonstrated
as a secure and reliable technique to deal with these issues offering the strengths of lesion localization even in proximal terminal
and preoperative guidance [2]. It is also showed that for the malignancy diagnosis the positive predictive value of ductoscopy is
reported up to 41% following the highest value of mammography
while its negative predictive value is exhibited as highest of 96%
which is the same value as MRI, but it is has the highest diagnostic
accuracy 88% than MRI in meta-analysis study with 3764 patients
in 36 studies included [18]. This finding is also corresponding to a
previous one of ductoscopy detection accuracy up to 94% for indicating malignancies in patients with PND [27]. Additionally, based
on one study of exploring the follow-on satisfaction of patients
with PND who has been performed with ductoscopy successfully
has gained good result as an important predictor and even no impact on the quality of life of patients in PND over time [28].
Magnetic resonance imaging
MRI has been mostly recommended in recent studies as an
emerging and preferred diagnosis instrument for screening highrisk patients with PND or detecting the primary origin of carcinoma [22,30] due to its strengths of being less invasive and no
radiation especially when the findings of first-line imaging modalities of mammography and ultrasound are normal but the PND
symptoms are not in resolution. Based on previous review, both
of mammography and ultrasound techniques have limitations
such as low sensitivity in etiology detection in terms of small lesions, not in calcifications conditions and locating retroareolar
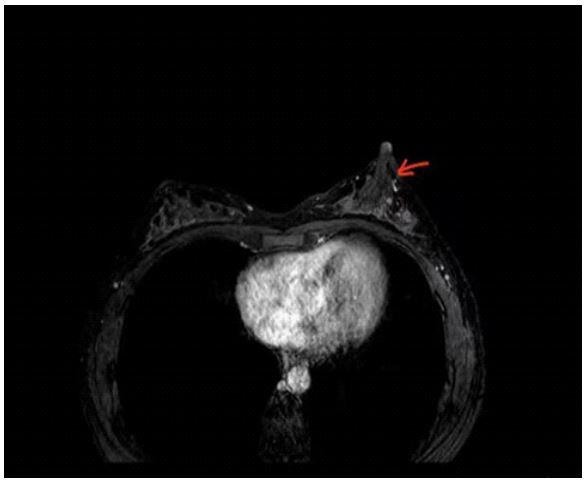
areas even its extent of scope [22,30,31]. From this, MRI can detect non-mass and mass enhancement (Figure 5) then even further conduct subcutaneous MRI-guided biopsy for histopathological diagnosis to rule out malignancy if surgery is essential [15].
According to Filipe et al. study of collecting 36 studies with 3764
patients in a meta-analysis regarding the detection of malignancy
in patients with PND with diagnostic imaging techniques, MRI
has shown the highest average sensitivity among others such as
ductoscopy, ultrasound, cytology, and mammography of 83% followed by ductoscopy of only 58%, its specificity is 76%, positive
predictive value is 40% while negative predictive value is highest
as the same as ductoscopy of 96% with its diagnostic accuracy
77% [18]. Its highest diagnostic sensitivity for detecting the malignant probability of patients of PND has been demonstrated in
many other types of research Nakahara et al. reported that MRI
sensitivity is 100%, while among 22 malignancies it has demonstrated 7 cases of malignant lesions (4 DCIS) which other imaging
modalities have not uncovered [30]; Lorenzon et al. has also stated that its statistically significant highest in sensitivity of 94.7%
which malignant lesions only detected via MRI [32]. In Bahl et al.
retrospective study of MRI in the evaluation of patients of PND,
its sensitivity of 100% and specificity of 65% as well as NPV of
100% of MRI, has stated its promising reliability and is widely recognized [33]. Many other studies have illustrated MRI’s highest
sensitivity and NPV regarding PND syndromes [34-36]. The predictive values of PPV and NPV of MRI for high-risk lesions and carcinoma are highest at 56% and 87%, respectively, in Morrogh et al.
study, which almost corresponds to the meta-analysis study [37].
However, its limitation has fallen into its higher cost and the false
positive results which are reflected in case of incidental lesions
unrelated to initial PND complaint as well as determining whether
the lesion is incurring intraductal that more imaging diagnosis of
mammography or ultrasound in a second look or ductoscopy detection are further indispensable [22,38].
Surgical techniques: The pioneering technique diagnosis and
treatment of PND.
Surgical anatomy: Breasts consist primarily of fatty tissue and
parenchyma, located between the anterior chest wall superficial
fascia and pectoralis central fascia [40]. There are 5-9 ductal orifices in the nipple central and peripheral region while 18-25 ducts
behind the nipple-areolar complex, according to surgical anatomical cross-sectional studies, but merely 8-12 ductal orifices are lactating. Most are truncated without forming into formal lactiferous
ducts connected with branching glands and generally terminated
with a sebaceous gland near the areolar area. Larger ducts are
distributed and branched widely into different quadrants within
breast tissues. Optimizations of surgical approaches by surgeons
could be facilitated by a more comprehensive realization of breast
anatomy [41].
Anesthesia: General anesthesia or intravenous sedation for
local anesthesia can be performed preoperative duct excision,
given the nerve sensitivity of the nipple-areolar complex. Additionally, proper risk screening should be conducted before general
anesthesia in patients [42].
Localization: Imaging modalities can be of direction to localize
the lesions such as mammographic, ultrasound, galactographic,
or ductoscopic guidance, among which intraoperative ultrasound
localization or preoperative ductoscopy with an injection of the
mixture of methylene blue and radiopaque dye are recommended
that could provide a direct visualization imaging of stained lesions
for better identification and operation [43-45].
Patients diagnosed with benign papilloma diseases without
atypia transformation in biopsy could have a low-risk rate of suffering from malignant results; observations could be alternative to
surgical treatment. However, in case of any risks of breast cancer
occurrence, the corresponding treatments are essential required,
for instance, drainage of the fluid-filled cyst via Fine Needle Aspiration (FNA), surgical operations via Vacuum-Assisted Breast Biopsy (VABB) of removing lumps and oral antibiotics administration
of infectious breast inflammation [46].
Vacuum-Assisted Breast Biopsy (VABB) is a more novel and modernized technique for biopsy, diagnosis, and treatment related
to breast diseases which were initially invented and developed
by a radiologist named Fred Burbank and his colleague medical
engineer Mark Retchard in 1995 in an attempt to overcome the
weakness and increase the accuracy of core biopsies such as wellestablished Fine-Needle Aspiration (FNA) and Core-Needle Biopsy
(CNB) that were popular in the 1980s and 1990s. One year later,
VABB was created, and Burbank and his other colleague Parker
introduced the stereotactic VABB as a diagnostic device for the
evaluation of breast lesions visibly with the auxiliary imaging modality mammography [47,48]. Then in 1998, Zannis first conducted ultrasound-guided VABB, and gradually even MRI-guided
VABB has been quantitatively applied to practical cases so far,
both of which have avoided the possibility of radiation in terms of
imaging modalities while the current equipment commonly used
in intraoperative employment are Mammotome® VABB, SenoRx
EnCor® VABB and XiShan Rotary® DK-B-MS. Additionally, compared to FNA or CNB and even conventional open surgical excision, there are several studies have exhibited and stated that VABB obtains higher diagnostic accuracy as a safe, superior, and valuable
technique for benign breast diseases and early breast cancer (ACR
BI-RADS® III-IVa) for treating patients in PND, which is an ideal
technique for diagnosis and therapeutic value [49-51]. Some studies employing ultrasound-guided VABB still have gained excellent results of more security, diagnostic effectiveness, and better
prognosis [49,50]. In accordance with the meta-analysis research
of Ding et al., including 15 types of research of 5256 patients who
have been performed Mammotome-VABB and conventional surgical excision regarding the factors of the incision or scar size scar,
operative time, wound recovery time, breast deformation and intraoperative hemorrhaging that VABB has demonstrated the apparent merits [51].
VABB system has three mainstream categories: stereotactic
VABB, ultrasound-guided handheld VABB, and Magnetic Resonance Imaging (MRI)-guided VABB. It is a minimally invasive surgical technique composed of two structural modules: A vacuum
pump and a rotary cutter. The vacuum pump is controlled by a
computer software device that can maintain negative pressure to enable the rotational cutter to make suction on the lesion part and
then facilitate biopsy sample collection automatically or manually.
At the same time, a rotary cutter is designed with a hollow lumen
with a groove that sucks on the corresponding resected lesions
and completes the rotary excision process. VABB mainly contains
three types of needles of different diameters that have been approved by the American Food and Drug Administration (FDA):
8G (250-310 mg), 11G (83-116 mg), or 14G (40 mg, twice than
conventional biopsy gun) of which the most appropriate type is
more dependent on the size of breast lesion since the volume differs then collecting sampling tissues varies each time with a single
insertion. The most put into clinical application is ultrasound-guided VABB throughout the entire surgical process (Figure 7); the
size of the skin incision merely needs 3-5 mm via puncturing the
probe into the skin to reach the target lesion dyed in preoperative
staining subcutaneously simultaneously guided by sonography
positioning which could be access to the visualization underneath
via high-resolution image on the monitoring screen. The features
of accuracy and efficiency could be achieved via this device, thus
reducing the possibility of sampling errors [48,50,51].
Discussion
Patients who have suffered from Pathological Nipple Discharge
(PND) are required to obtain medical history primarily and perform physical breast examinations, given that factors are related to the probability of prognostic complications or worsening
conditions. In contrast, physical examinations are required to detect breast symmetry or contour and noticeable physical changes
like edema if there is a palpable breast mass [15]. If clinical manifestations of bloody discharge unilaterally and spontaneously
still occur following the front-line diagnostic imaging modalities
of mammography or routine ultrasound combined with ContrastEnhanced Ultrasound (CEUS) to investigate the abnormalities or
normalities and determine the BI-RADS for the early detection of
benign lesions and malignant etiology. If there are normal conditions for which reassurance should be followed up or further to
the next step for more auxiliary diagnosis to avoid false negative
rate. The second-line imaging technique, such as ductoscopy, is for
the visualization of intraductal conditions to define the etiology.
At the same time, MRI is highly recommended to perform due to
its highest average sensitivity for patients, particularly those who
have not been investigated commonly in previous imaging modalities [18]. Selecting the most suitable diagnostic methods is required for the best strategy for diagnosis and treatment. Referring
to the surgeon for surgical treatment has gained popularity for
fewer residues of lesion removal and a more significant amount
of contiguous samples collection via modernized Vacuum-Assisted Breast Biopsy (VABB) of ultrasound guidance via 8G or 11 G
needle compared to 14 G and open conventional surgical incision
to further diagnosis in histopathological examination in the reconfirmation of etiology and active measures for postoperative
treatment if the detection is malignancy [52].
Conclusion
Initiating a thorough essential clinical evaluation of historical
inquiry and physical examination enable clinicians to get first-hand
materials of patients manifesting conditions of nipple discharge.
The imaging techniques are essential in the early diagnosis and detection in patients of Pathological Nipple Discharge (PND) preoperatively. Surgical intervention for the treatment of a sonography-guided Vacuum Assist Breast Biopsy (VABB) for lesions removal and specimens offering is a therapeutic, surgical technique of
high efficiency, safe desirability, and valuable practicality.
Declarations
Conflict of interest statement: The authors have no conflicts of interest to declare.
Funding sources: The author did not receive funds.
Acknowledgment: As a result, we acknowledge the people
mentioned above who have offered supportive assistance in our
work from writing spur, which authors acknowledge.
References
- Román M, Louro J, Posso M, et al. Breast density, benign breast disease, and risk of breast cancer over time. Eur Radiol. 2021; 31: 4839-47.
- Hussain AN, Policarpio C, Vincent MT. Evaluating nipple discharge. Obstet Gynecol Surv. 2006; 61: 278-83.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006; 72: 124.
- Nelson RS, Hoehn JL. Twenty-year outcome following central duct resection for bloody nipple discharge. Ann Surg. 2006; 243: 522.
- Kooistra BW, Wauters C, van de Ven S, Strobbe L. The diagnostic value of nipple discharge cytology in 618 consecutive patients. Eur J Surg Oncol. 2009; 35: 573.
- King TA, Carter KM, Bolton JS, Fuhrman GM. A simple approach to nipple discharge. Am Surg. 2000; 66: 960.
- Murad TM, Contessa G, Mouriesse H. Nipple discharge from the breast. Ann Surg. 1982; 195: 259.
- Jardines L. Management of nipple discharge. Am Surg. 1996; 62: 119.
- D’Alfonso TM, Ginter PS, Shin SJ. A review of inflammatory processes of the breast with a focus on diagnosis in core biopsy samples. Journal of pathology and translational medicine. 2015; 49: 279.
- Golshan M, Iglehart D. Nipple discharge. Upto Date. Philadelphia, PA: Wolters Kluwer Health. 1992.
- Kamali S, Bender O, Kamali GH, Aydin MT, Karatepe O, et al. Diagnostic and therapeutic value of ductoscopy in nipple discharge and intraductal proliferation compared with standard methods. Breast Cancer. 2014; 21: 154-161.
- Dixon JM. Periductal mastitis/duct ectasia. World J Surg. 1989; 13: 715-720.
- Collins LC, Laronga C, Wong JS, Pierce LJ, Dizon DS, et al. Breast ductal carcinoma in situ: Epidemiology, clinical manifestations, and diagnosis. 2017.
- Sakorafas GH. Nipple discharge: Current diagnostic and therapeutic approaches. Cancer treatment reviews. 2001; 27: 275-82.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. The American journal of medicine. 2015; 128: 353-60.
- Lee SJ, Trikha S, Moy L, Baron P, Green ED, et al. ACR Appropriateness Criteria® evaluation of nipple discharge. Journal of the American College of Radiology. 2017; 14: S138-53.
- Wentz G, Parsons WC. Mammography for radiologic technologists. McGraw-Hill, Health Professions Division. 1997.
- Filipe MD, Patuleia SI, de Jong VM, Vriens MR, van Diest PJ, et al. Network Meta-analysis for the Diagnostic Approach to Pathologic Nipple Discharge. Clinical Breast Cancer. 2020; 20: e723-48.
- D’Orsi C, Bassett L, Feig S. Breast imaging reporting and data system (BI-RADS). Breast imaging atlas. 2018.
- Rao AA, Feneis J, Lalonde C, Ojeda-Fournier H. A Pictorial Review of Changes in the BI-RADS Fifth Edition. Radiographics. 2016; 36: 623-39.
- Bahl M, Baker JA, Greenup RA, Ghate SV. Diagnostic value of ultrasound in female patients with nipple discharge. American Journal of Roentgenology. 2015; 205: 203-8.
- Paula IB, Campos AM. Breast imaging in patients with nipple discharge. Radiologia brasileira. 2017; 50: 383-8.
- Zhao LX, Liu H, Wei Q, Xu G, Wu J, et al. Contrast-enhanced ultrasonography features of breast malignancies with different sizes: Correlation with prognostic factors. Bio Med research international. 2015.
- Balci FL, Feldman SM. Interventional ductoscopy for pathological nipple discharge. Annals of surgical oncology. 2013; 20: 3352-4.
- Filipe MD, Patuleia SI, Vriens MR, van Diest PJ, Witkamp AJ. Meta-analysis and cost-effectiveness of ductoscopy, duct excision surgery and MRI for the diagnosis and treatment of patients with pathological nipple discharge. Breast cancer research and treatment. 2021; 1-9.
- Kapenhas-Valdes E, Feldman SM, Boolbol SK. The role of mammary ductoscopy in breast cancer: a review of the literature. Annals of surgical oncology. 2008; 15: 3350-60.
- Waaijer L, Simons JM, Borel Rinkes IH, Van Diest PJ, Verkooijen HM, et al. Systematic review and meta-analysis of the diagnostic accuracy of ductoscopy in patients with pathological nipple discharge. Journal of British Surgery. 2016; 103: 632-43.
- Filipe MD, Simons JM, Moeliker L, Waaijer L, Vriens MR, et al. Patient-reported outcomes of ductoscopy procedures for pathologic nipple discharge. Breast Cancer. 2021; 28: 471-7.
- Denewer A, El‐Etribi K, Nada N, El‐Metwally M. The role and limitations of mammary ductoscope in management of pathologic nipple discharge. The breast journal. 2008; 14: 442-9.
- Nakahara H, Namba K, Watanabe R, et al. A comparison of MR imaging, galactography and ultrasonography in patients with nipple discharge. Breast Cancer. 2003; 10: 320-329.
- Ballesio L, Maggi C, Savelli S, et al. Role of breast Magnetic Resonance Imaging (MRI) in patients with unilateral nipple discharge: preliminary study. Radiol Med. 2008; 113: 249-264.
- Lorenzon M, Zuiani C, Linda A, et al. Magnetic resonance imaging in patients with nipple discharge: Should we recommend it? Eur Radiol. 2011; 21: 899-907.
- Bahl M, Baker JA, Greenup RA, Ghate S V. Evaluation of Pathologic Nipple Discharge: What is the Added Diagnostic Value of MRI? Ann Surg Oncol. 2015; 22: S435-41.
- Gupta D, Mendelson EB, Karst I. Nipple discharge: Current clinical and imaging evaluation. American Journal of Roentgenology. 2021; 216: 330-9.
- Zacharioudakis K, Kontoulis T, Vella XJ, Zhao J, Ramakrishnan R, et al. Can we see what is invisible? The role of MRI in the evaluation and management of patients with pathological nipple discharge. Breast Cancer Res Treat. 2019; 178: 115-20.
- Lubina N, Schedelbeck U, Roth A, et al. 3.0 Tesla breast magnetic resonance imaging in patients with nipple discharge when mammography and ultrasound fail. Eur Radiol. 2015; 25: 1285-1293.
- Morrogh M, Morris EA, Liberman L, Borgen PI, King TA. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007; 14: 3369-77.
- Bicchierai G, Di Naro F, De Benedetto D, Cozzi D, Pradella S, et al. A Review of Breast Imaging for Timely Diagnosis of Disease. International Journal of Environmental Research and Public Health. 2021; 18: 5509.
- Bahl M, Gadd MA, Lehman CD. JOURNAL CLUB: Diagnostic Utility of MRI after Negative or Inconclusive Mammography for the Evaluation of Pathologic Nipple Discharge. AJRAmerican J Roentgenol. 2017; 209: 1404-10.
- Reynolds A. Stereotactic breast biopsy: A review. Radiol Technol. 2009; 80: 447M.
- Love SM, Barsky SH. Anatomy of the nipple and breast ducts revisited. Cancer. 2004; 101: 1947.
- Williams FM, Bergin JD. Cardiac screening before noncardiac surgery. Surg Clin North Am. 2009; 89: 747.
- Kaufman CS, Jacobson L, Bachman B, Kaufman LB. Intraoperative ultrasonography guidance is accurate and efficient according to results in 100 breast cancer patients. Am J Surg. 2003; 186: 378.
- Rahusen FD, Bremers AJ, Fabry HF, et al. Ultrasound-guided lumpectomy of nonpalpable breast cancer versus wire-guided resection: a randomized clinical trial. Ann Surg Oncol. 2002; 9: 994.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003; 196: 354.
- Moynihan A, Quinn EM, Smith CS, Stokes M, Kell M, et al. Benign breast papilloma: Is surgical excision necessary? The breast journal. 2020; 26: 705-10.
- Burbank F. Mammographic findings after 14-gauge automated needle and 14-gauge directional, vacuum assisted stereotactic breast biopsies. Radiology. 1997; 204: 153-6.
- Park HL, Hong J. Vacuum-assisted breast biopsy for breast cancer. Gland surgery. 2014; 3: 120.
- Xu Y, Ming J, Zhou Y, Qi X, Fan L, et al. Mammotome-assisted endoscopic breast-conserving surgery: A novel technique for earlystage breast cancer. World journal of surgical oncology. 2014; 12: 1-6.
- Torres-Tabanera M, Alonso-Bartolomé P, Vega-Bolivar A, SánchezGómez SM, Lag-Asturiano E, et al. Percutaneous microductectomy with a directional vacuum-assisted system guided by ultrasonography for the treatment of breast discharge: Experience in 63 cases. Acta Radiologica. 2008; 49: 271-6.
- Ding B, Chen D, Li X, Zhang H, Zhao Y. Meta-analysis of efficacy and safety between Mammotome vacuum-assisted breast biopsy and open excision for benign breast tumor. Gland surgery. 2013; 2: 69.
- Parker SH, Klaus AJ. Performing a breast biopsy with a directional, vacuum-assisted biopsy instrument. Radiographics. 1997; 17: 1233-52.