Introduction
Nivolumab, a monoclonal anti-PD-1 (programmed death 1)
antibody, interferes with PD-ligand 1-mediated signaling and restores the immune system’s anti-tumor defenses. In Japan, nivolumab was approved for the treatment of Renal Cell Carcinoma
(RCC) after several agents such as sunitinib, axitinib, sorafenib and
everolimus, and has been widely used as a third-line or later treatment [1]. Immune-Checkpoint Inhibitors (ICIs) can cause various
immune-related Adverse Events (irAEs), including Interstitial Lung
Disease (ILD) called as Checkpoint Inhibitor Pneumonitis (CIP).
Although most irAEs are generally reversible with immunosuppressive treatments, several retrospective studies have reported
refractory CIP [2,3]. Here, we experienced a case of steroid-dependent CIP that relapsed multiple times over three years after
discontinuation of nivolumab.
Case report
A 66-year-old woman who had never smoked and had postoperative recurrent RCC with retroperitoneal local recurrence, multiple abdominal lymph nodes, and bone metastases was treated
sequentially with sunitinib for approximately 3 months, everolimus for 2 weeks, and axitinib for 1 month. However, her disease
progressed. In November 2018, she started receiving nivolumab
200 mg every 3 to 4 weeks and achieved a complete response.
She complained of a cough and shortness of breath after receiving 13 doses of nivolumab in August 2019 and was admitted to our hospital. Her oxygen saturation on room air was 96%. Fine
crackles were audible in both lung fields without fever or signs of
arthritis or skin lesions. Laboratory tests showed a white blood
cell count of 8,100 /μL, C-Reactive Protein (CRP) level of 22.0 mg/
dL (normal range: <0.3 mg/dL), Lactate Dehydrogenase (LDH) level of 195 IU/L (normal range: 110-224 IU/L), Sp-D level of 229 ng/
mL (normal range: <110 ng/mL) and KL-6 level of 131 U/mL (normal range: <500 U/mL). Anti-aminoacyl-tRNA synthetase (ARS)
antibody, anti-nuclear antibody, proteinase 3(PR-3) - and Myeloperoxidase (MPO) antineutrophil cytoplasmic antibodies were
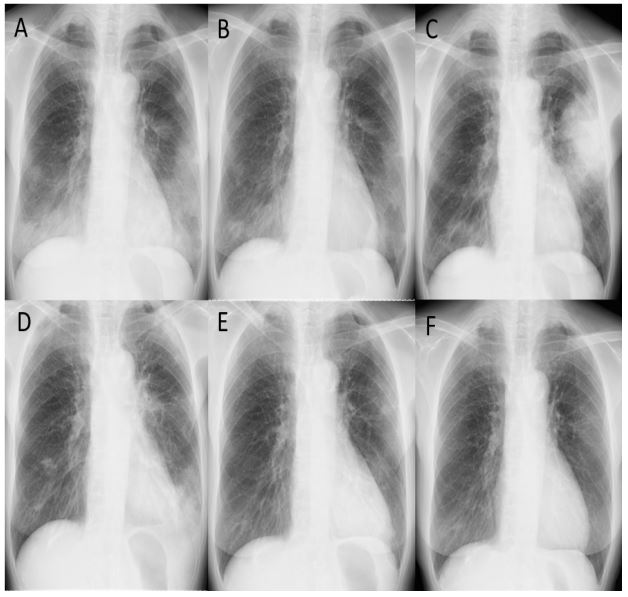
negative. Chest X-ray showed reticular opacities in the bilateral
lower lung fields (Figure 1A), and Computed Tomography (CT) of
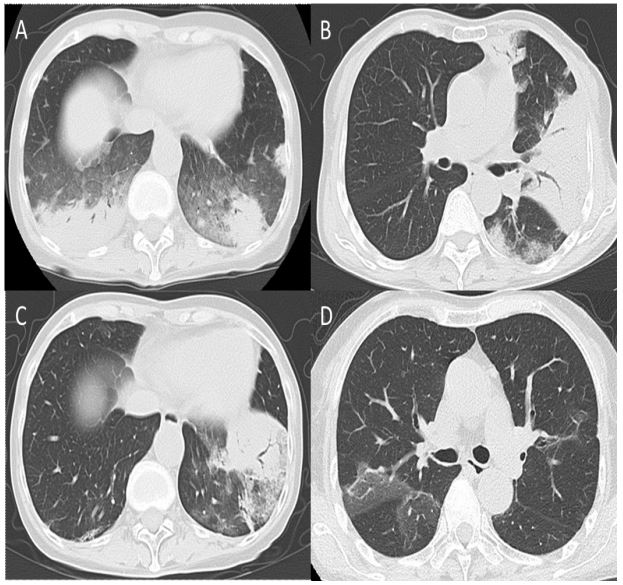
the chest revealed the presence of multiple consolidations with
bronchiectasis and surrounding Ground-Glass Opacities (GGOs) in
both lungs (Figure 2A). These findings are consistent with a Cryptogenic Organizing Pneumonia (COP)-like pattern in the radiographic patterns of ILD. Her sputum and bronchial lavage fluid (left
B9) showed no bacteriological findings. Pathological findings of a
transbronchial lung biopsy specimen (left B9) revealed organizing
pneumonia leading to a diagnosis of CIP with CTCAE Grade 2. Nivolumab was discontinued and she received steroid pulse therapy
(methylprednisolone 1000 mg for 3 days), followed by prednisolone (PSL) 40 mg/day (1 mg/kg/day). Radiographic findings improved significantly six days after the initiation of steroid therapy
(Figure 1B), and the steroid dose was gradually reduced over 15
weeks. Five days after achieving complete remission of CIP and
completing the PSL taper, she complained of chest pain and fever.
Influenza antigen test was negative and laboratory tests showed
a white blood cell count of 9,600 /μL (neutrophils: 81.9%, eosinophils: 2.7%, lymphocytes: 9.1%), CRP level 25.8 mg/dL, LDH level
190 IU/L, Sp-D level 144 ng/mL, KL-6 level 113 U/mL procalcitonin level 0.11 ng/mL (normal range: <0.11 ng/mL), and βD glucan
level 7.6 pg/mL (normal range: <20 pg/mL). She was diagnosed
with pneumonitis again and treated with antibiotic therapy (tazobactam/piperacillin hydrate), but her condition did not improve.
She was diagnosed with a recurrence of CIP (Figure 1C, 2B) and
was readministered PSL 40 mg/day after steroid pulse therapy,
which resulted in a remarkable improvement. Ten days after PSL
was tapered down to 5.0 mg/day more slowly in April 2020, she
complained of chest discomfort and experienced a second recurrence of CIP (Figure 1D, 2C). PSL was increased to 30 mg/day and
then tapered to 10 mg/day over 6 months. In April 2021, a CT scan
demonstrated GGOs in the right upper and middle lobes (Figure
2D), suggesting asymptomatic CIP, which resolved without requiring an increase in the steroid dose. After tapering the PSL dose
to 7.5 mg/day in November 2022, the patient experienced a recurrence of CIP, resulting in dyspnea in January 2023. (Figure 1E),
and the PSL dose was increased back to 10 mg/day. Since then,
there have been no further recurrences of CIP while the patient
has been receiving a PSL dose of 10 mg/day (Figure 1F).
Discussion
Nivolumab is known to occupy PD-1 on T cells for several hundred days after the last dose [4], and the immunological activation
induced by PD-1 blockade would be expected to persist long-term
even after removal of nivolumab. In this case, complete remission
of RCC was sustained long after discontinuation of nivolumab, during which time CIP recurred multiple times. This may be related
to the sustained immunostimulatory effects of ICI and the association between ICI efficacy and irAEs [5].
Several reports have shown that CIP does not respond to corticosteroid treatment or persists long after immunotherapy is
discontinued [2,3]. Naidoo J et al. demonstrated that 14% of CIP
persisted beyond 12 weeks after the cessation of ICI and became
chronic despite adequate immunosuppression [6]. In our case,
the exact reason for multiple unprovoked recurrences of CIP is
unknown. After discontinuation of nivolumab, RCC has not recurred and no new drugs have been administered, such as other
anticancer drug treatments that could cause drug-induced lung
injury. Whenever pneumonitis recurred, we did not perform a
histological examination using bronchoscopy or measure viral antigens and various autoantibodies to proactively investigate other
causes of recurrent pneumonitis, such as infections or collagen diseases. However, each time the patient experienced a recurrence
of pneumonia, they complained of symptoms similar to those of
the initial pneumonia, rather than symptoms of upper respiratory
infection such as a sore throat, or symptoms of collagen vascular
disease such as joint and muscle pain. The patient had undergone
several therapies before receiving nivolumab, including everolimus, which has a higher incidence of drug-induced interstitial
lung disease compared to nivolumab. However, the initial pneumonitis occurred after 10 months of receiving nivolumab, and we
do not believe it was caused by other RCC drugs administered
prior to nivolumab, such as axitinib or everolimus. We suspected
that the effect of prior therapies could be one of the possible mechanisms for the repeated recurrences in this patient. However,
recent reports have shown that prior therapy does not increase
the incidence of nivolumab-induced irAEs in RCC patients [1,7].
A Guideline for the treatment of irAEs states that corticosteroids should be tapered over the course of at least 4-6 weeks [8].
Tao H. et al. conducted a cohort study of CIP recurrence in lung
cancer patients, and showed that the duration of PSL equivalent
dose ≥15 mg/day in patients with CIP recurrence was significantly
shorter than in patients without recurrence [9]. They recommend
administration of a PSL-equivalent dose ≥15 mg/day at least 4
weeks to prevent recurrence of CIP. In addition, it is known that
ILD with a radiographic COP-like pattern often recurs when corticosteroid doses are tapered or discontinued, and corticosteroid
should be tapered more slowly in recurrent cases. In our case,
duration of PSL ≥15 mg/day in initial CIP, first and second CIP recurrence was 9.2, 9.2, and 17.7 weeks, respectively, which are
considered sufficient corticosteroid doses and durations.
Most irAEs resolve with a corticosteroid taper of 4-8 weeks,
but refractory cases may require immunosuppressive therapy
other than corticosteroids. There is no consensus on the treatment of steroid-refractory CIP, which is defined as no improvement of respiratory symptoms within 72 hours of appropriate
corticosteroid therapy. In practice, the following drug options are
used: infliximab, mycophenolate mofetil, tocilizumab, and Intravenous Immunoglobulin (IVIG) [2,3,10]. In our case, PSL 10 mg/
day was administered long-term after a second recurrence of CIP,
which occurred while on PSL 5 mg/day. Given the side effects of
long-term corticosteroid therapy, PSL was reduced to 7.5 mg/day,
resulting in a relapse. Chronic steroid-dependent CIP may require
the addition of a second immunosuppressive agent for steroid
weaning, as well as for steroid-refractory/resistant CIP.
We present a case of chronic steroid-dependent CIP with multiple recurrences over three years after discontinuation of ICI. Careful observation is required when tapering corticosteroid doses,
even long after discontinuation of ICI. Further research is needed
on the risk factors for chronic steroid-dependent CIP and the development of appropriate management strategies.
Declarations
Conflict of interest statement: The authors have no conflicts of interest.
Ethics statement: Written informed consent was obtained
from the patient for publication of this case report.
References
- Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 Check Mate 025 trial. Cancer. 2020; 126: 4156-4167.
- Beattie J, Rizvi H, Fuentes P, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer. 2021; 9: e001884.
- Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: Incidence, clinical features, treatment, and outcomes. J Immunother Cancer. 2021; 9: e001731.
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010; 28: 3167-75.
- Cui P, Huang D, Wu Z, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol. 2020; 12: 1758835920922033.
- Naidoo J, Cottrell TR, Lipson EJ, et al. Chronic immune checkpoint inhibitor pneumonitis J Immunother Cancer. 2020; 8: e000840.
- Mizutani K, Ito T, Takahara K, et al. Frequency of pre-treatment may not increase the immune-related adverse events of RCC patients treated with nivolumab. Medicine (Baltimore). 2021; 100: e25402.
- Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol. 2021; 39: 4073-4126.
- Tao H, Li F, Wu D, et al. Rate and risk factors of recurrent immune checkpoint inhibitor-related pneumonitis in patients with lung cancer. Transl Lung Cancer Res. 2022; 11: 381-392.
- Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019; 25: 551-557