Introduction
Intracranial epidermoid cysts are rare but generally benign intracranial tumours [1,2].
These cysts can undergo malignant transformation into squamous cell carcinoma (SCC), but only a few such cases have been
described since the first report in 1912 [1]. Intracranial SCC may
originate from a known intracranial epidermoid cyst or it may be
a de novo tumour [3].
Here we report a case of primary intracranial SCC. In addition,
we review the literature on this rare tumour type.
Case report
History and examination
A 42-year-old woman presented with a 6-month history of
right-side deafness and a 5-month history of dizziness and tinnitus. She had no relevant medical history.
Imaging studies
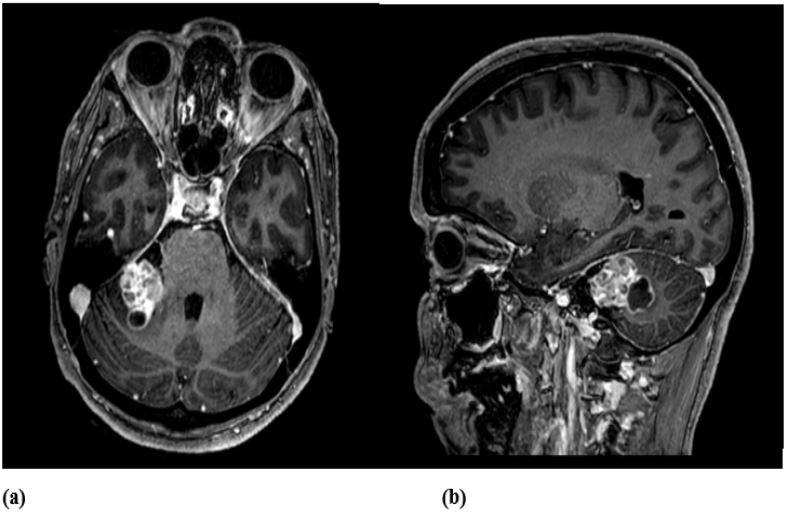
Contrast-enhanced Computed Tomography (CT) revealed a lesion with multicystic heterogeneous enhancement and Magnetic
Resonance Imaging (MRI) showed a right Cerebellopontine Angle
(CPA) mass invading the right inner auditory canal (Figure 1). These radiological features, together with the clinical presentation, mimicked a schwannoma of cranial nerve VIII.
Surgical treatment and postoperative course
A right retrosigmoid craniotomy with gross total resection of
the tumour was performed on November 13, 2019. Intraoperatively, the macroscopic appearance of the tumour was heterogenous, consisting of fibrous, highly vascularized areas alternating
with necrotic and cystic areas. The tumour was indistinguishable
from an acoustic schwannoma. The postoperative course was
uneventful and the patient was discharged home with no new
neurological deficits. During clinical follow-up, the dizziness decreased and the tinnitus resolved, but the ipsilateral hypoacusis
remained unchanged.
Pathological findings
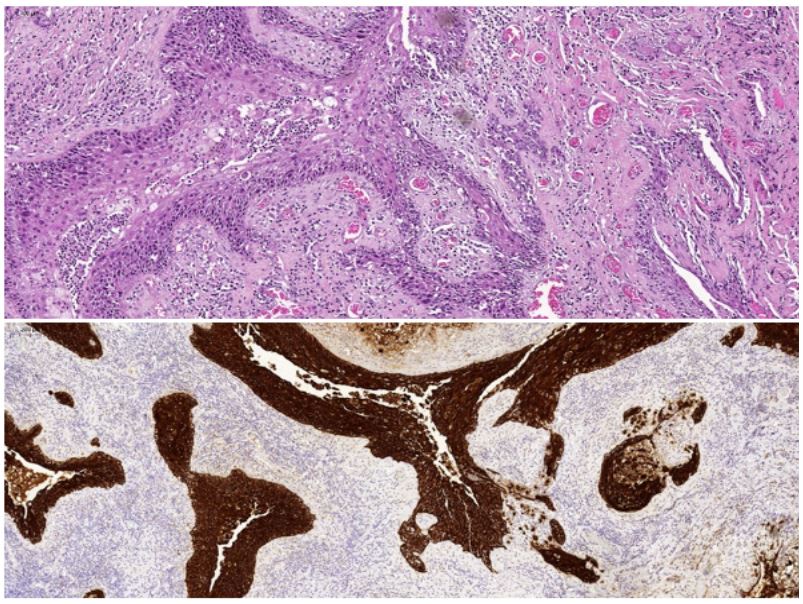
Histologic examination of the resected specimen revealed a
solid-cystic lesion lined by squamous epithelium with moderate
atypia and abundant mitotic figures consistent with SCC (Figure
2). These features were suggestive of a primary central nervous
system SCC arising from a pre-existing epidermoid cyst.
To rule out the presence of an extracranial primary tumour,
Positron Emission Tomography (PET) was performed. The results
of this scan were normal.
Radiation therapy
This case was presented to a multidisciplinary tumour board,
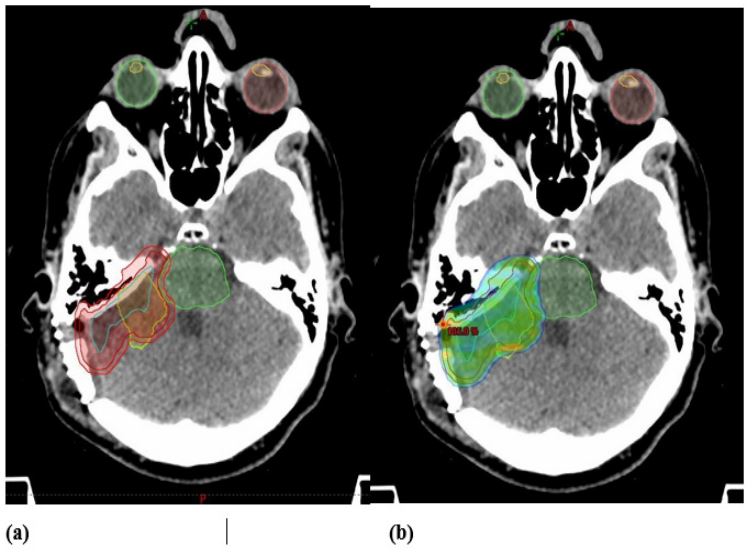
which decided that the patient was a candidate for adjuvant radiotherapy. Fractionated stereotactic radiotherapy was prescribed (50 Gy in 25 fractions) (Figure 3). The treatment was well-tolerated and the final session was delivered in late February 2020.
Treatment outcomes
An MRI performed in June 2020 (7 months after surgery) revealed focal enhancement in the surgical margin on the right cerebellar peduncle and a thin enhancement in the right internal
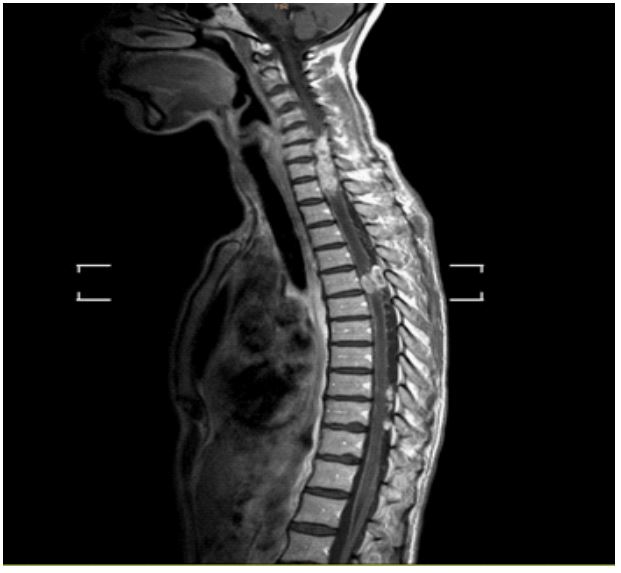
acoustic canal. On a follow-up MRI (January 2022), the previous
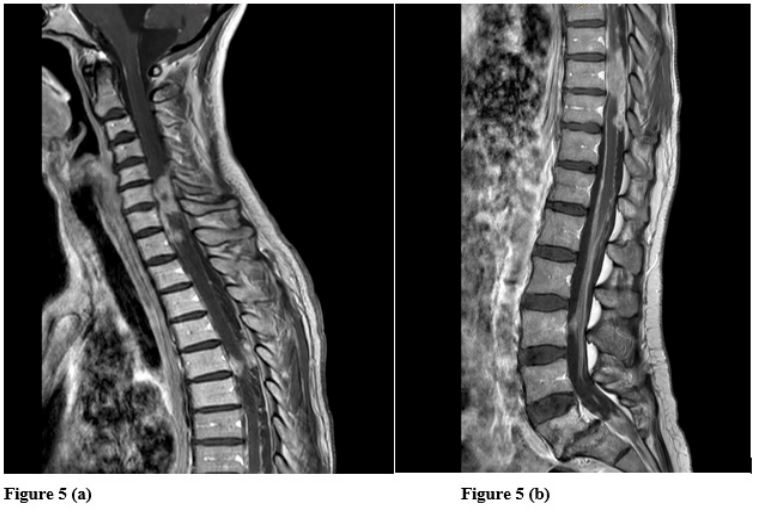
findings remained stable, but revealed the presence of leptomeningeal progression in the cervical and thoracic spinal cord, resulting in spinal cord compression (Figure 4a,b). Consequently, local
radiotherapy (30 Gy in 10 fractions) was administered to that region. The patient started intrathecal treatment with methotrexate
in March 2022. A follow-up MRI (April 2022) revealed leptomeningeal progression with secondary spinal cord compression from T9
to T11. Another course of radiotherapy was prescribed (30 Gy).
However, the patient did not complete radiotherapy (receiving
only 6 Gy) (Figure 5a,b) and died on May 5, 2022 due to neurological impairment.
Methods and results
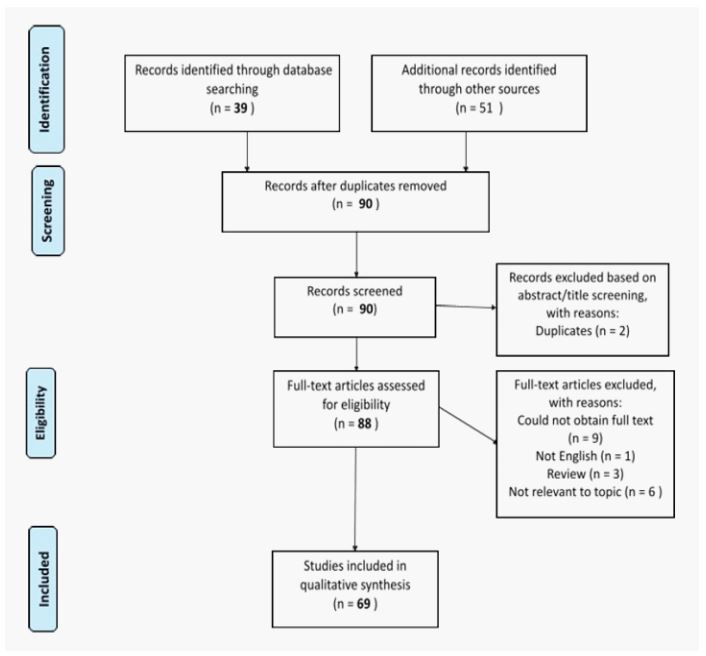
We performed a systematic review of the literature using the
criteria established in the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA). We searched the
PubMed and Medline databases for relevant articles published
through March 31, 2021 using the following search terms were
used: “intracranial squamous cell carcinoma” OR “intracranial
epidermoid cyst” OR “malignant transformation of intracranial
epidermoid cyst”. The inclusion and exclusion criteria are shown in Figure 6.
A total of 90 articles were identified for further review. Of
these, 69 case reports describing secondary malignant transformation of intracranial epidermoid cysts were identified (Table 1).
Most of the patients described in these case reports were males
(n=41; 59.4%). The mean age was 52.5 years (range, 20-77). Six of
the cases were diagnosed during the autopsy.
In most cases (61/69; 88.4%) surgery was the primary treatment. Thirty-one patients received adjuvant radiotherapy
(conventional radiotherapy, radiosurgery, or proton beam therapy), with or without chemotherapy.
Apart from the current case, a total of ten cases involving primary intracranial SCC have been reported to date (Table 2). The
most commonly reported location is the CPA (5/10 cases), which
was the same area as secondary malignant carcinomas. Most of
these patients (8/10) underwent surgery, with four also receiving
adjuvant radiation therapy. Of the two nonsurgical patients, one
received definitive radiotherapy and the other was diagnosed at
autopsy.
Discussion
Intracranial epidermoid cysts account for 0.2-1.8% of all intracranial tumours [2,3]. These tumours are generally slow growing
and histologically benign. Malignant transformation of an epidermoid tumour is rare and de novo intracranial SCC is even more
uncommon.
The first case of SCC was reported by P. Ernst in 1912. This case
involved a 52-year-old man diagnosed with SCC of the CPA arising
from a known epidermoid cyst [1]. Since that time, a total of 73
cases of malignant transformation of epidermoid cysts have been
reported [2,4]. Primary intracranial SCC was first described in
1976 by Wong et al. [5]. To our knowledge, only nine other cases
have been reported since [6-14].
Intracranial SCC appears to be more common in males
[6,15,16], although Lakhdar et al described a greater predominance in women [9].
Types
Hamlat et al. [16] classified SCC into five different categories
according to the type of malignant transformation, as follows: 1)
initial malignant transformation of an epidermoid cyst, which is
the most common type; 2) malignant transformation from a remnant epidermoid cyst; 3) malignant transformation with leptomeningeal carcinomatosis (LC); 4) SCC carcinoma arising from other
benign cysts; and 5) other malignancies arising in benign cysts.
Malignant transformation
Based on the available reports, malignant transformation can
occur anywhere from 3 months to 33 years from diagnosis of the
benign lesion [16], with a median interval of 24 months [15].
Diagnosis
The symptoms of SCC are non-specific, which is why diagnosis
can be challenging. Selection of the appropriate diagnostic tests
depends on the symptoms, which vary according to the tumour
location. Rapid progression of symptoms may be an important clinical sign of malignant transformation [6].
Table 1: Reported cases of malignant transformation from epidermoid tumours.
| Author and year |
Age (Sex) |
Location |
Treatment |
Dose |
Survival status at last
evaluation
(time from
surgery)
|
Interval to MT,
months
|
| Ernst 1912 [1] |
52(M) |
CPA |
Autopsy |
|
|
NS |
| Hug 1942 [27] |
42(M) |
Parapontine |
Autopsy |
|
|
NS |
| Yamanaka 1955 [28] |
57(M) |
Base of brain |
Autopsy |
|
|
NS |
| Davidson 1960 [29] |
46(M) |
Frontal |
S + RT |
NS |
NS |
5 |
| Landers 1960 [30] |
73(F) |
Cerebellar |
Autopsy |
|
|
1 |
| Komjatszeg 1964 [31] |
45(F) |
Base of brain |
Autopsy |
|
|
NS |
| Toglia 1965 [32] |
54(F) |
Base of brain |
S |
|
NS |
12 |
| Fox 1965 [33] |
43(M) |
Temporal lobe |
S |
|
Deceased (1 month) |
84 |
| Dubois 1981 [34] |
53(M) |
Fourth ventricle |
S + RT |
50 Gy |
Deceased (2 months) |
NS |
| Takado 1982 [35] |
53(F) |
CPA |
Autopsy |
|
|
NS |
| Lewis 1983 [36] |
54(F) |
Parasellar |
S |
|
Deceased (1 month) |
36 |
| Giangaspero 1984 [37] |
45(M) |
Parieto-Occipital |
S + RT |
45 Gy |
Deceased (8 months) |
0.3 |
| Meffazoni 1986 [38] |
45(M) |
Frontal |
S |
|
NS |
NS |
| Kubokura 1986 [39] |
60(F) |
Temporal lobe |
S |
|
Deceased (8 days) |
NS |
| Goldman 1987 [40] |
59(F) |
Intraventricular |
S + RT |
50 Gy |
Alive (36 months) |
396 |
| Salazar 1987 [41] |
49(M) |
CPA + pons |
S + RT |
NS |
Alive (10 months) |
3 |
| Abramson 1989 [42] |
36(M) |
CPA |
S |
|
NS |
24 |
| Nishiura 1989 [43] |
38(M) |
CAP |
S + ChT |
|
Alive (24 months) |
6 |
| Gi 1990 [44] |
39(M) |
CPA |
S + RT |
50 Gy |
Deceased (15 months) |
6 |
| Tognetti 1991 [45] |
67(F) |
Temporal lobe |
S |
|
Deceased (1 month) |
372 |
| Acciarri 1993 [46] |
62(M) |
Parasellar |
S |
|
Deceased (1 week) |
2 |
| Nishio 1995 [47] |
57(M) |
CPA |
S + RT |
50 Gy |
Alive (30 months) |
12 |
| Nishio 1995 [47] |
42(M) |
Middle-posterior
fossa
|
S + RT |
60 Gy |
Deceased (3.5 months) |
NS |
| Uchino 1995 [48] |
57(M) |
CPA |
S + RT |
60 Gy |
Alive (4 months) |
18 |
| Ogata 1996 [49] |
63(F) |
Dorsolateral pons |
S |
|
Recurrence (3 months) |
NS |
| Mohanty 1996 [50] |
20(M) |
Posterior fossa |
S + RT |
NS |
Deceased (11 months) |
3 |
| Bayindir 1996 [51] |
67(F) |
Lateral ventricle |
S |
|
Alive (36 months) |
NS |
| Murase 1999 [52] |
50(F) |
CPA |
S + ChT + SRS |
14 Gy |
Alive (60 months) |
120 |
| Asahi 2001 [53] |
55(F) |
CPA |
S |
|
Deceased (3 months) |
66 |
| Nawashiro 2001 [24] |
46(M) |
Left temporal lobe |
S |
|
NS |
NS |
| Khan 2001 [54] |
53(M) |
Prepontine |
BSC |
|
Deceased (10 months) |
7 |
| Link 2002 [55] |
57(F) |
CPA |
S + RT + SRS |
45 Gy + 15 Gy |
Deceased (32 months) |
12 |
| Monaco 2003 [56] |
36(M) |
Cisterna magna |
S |
|
Alive (24 months) |
6 |
| Shirabe 2003 [57] |
49(M) |
Ventral pons |
S + RT |
60 Gy |
Deceased (42 months) |
18 |
| Hamlat 2003 [58] |
54(F) |
Parieto-temporal |
ChT |
Deceased (7 months) |
3 |
|
| Park 2003 [59] |
65(F) |
CPA |
S + RT |
50 Gy |
Alive (6 months) |
NS |
| Michael 2005 [60] |
45(M) |
Petroclival |
S + RT + ChT |
59.4 Gy |
Deceased (12 months) |
1 |
| Tamura 2006 [22] |
56(F) |
CPA |
S + SRS |
15 Gy |
Alive (13 months) |
96 |
| Kodama 2007 [61] |
67(M) |
CPA |
S + SRS |
14 Gy |
Deceased (11 months) |
NS |
| Pagni 2007 [62] |
65(F) |
Pineal |
S |
|
NS |
NS |
| Agarwal 2007 [63] |
45(M) |
Posterior fossa |
S |
|
NS |
NS |
| Kim 2008 [64] |
72(F) |
CPA |
S + RT |
54 Gy |
Alive (12 months) |
NS |
| Ge 2009 [65] |
50(M) |
Temporal love |
S |
|
NS |
72 |
| Hao 2010 [66] |
61(F) |
CPA |
S |
|
Deceased (1.2 months) |
36 |
| Nakao 2010 [67] |
74(F) |
CPA |
S + RT |
|
Alive (17 months) |
240 |
| Lakhdar 2011 [6] |
52(M) |
CPA |
S |
|
NS |
6 |
| Chon 2012 [68] |
43(M) |
CPA |
S + SRS |
25 Gy |
Alive (40 months) |
24 |
| Feng 2014 [69] |
42(M) |
CPA |
S + RT |
36 Gy |
Alive (6 months) |
- |
| Raheja 2016 [70] |
54(F) |
Prepontine |
S |
|
NS |
NS |
| Raheja 2016 [70] |
37(F) |
CPA |
S |
|
Deceased (17 months) |
NS |
| Pikis 2016 [23] |
77(M) |
CPA |
S + RT |
55 Gy |
Deceased (6 months) |
12 |
| Solanki 2016 [71] |
47(F) |
CPA |
S |
|
Deceased (6 weeks) |
12 |
| Ding 2016 [72] |
55(F) |
Temporal lobe |
S |
|
Deceased (12 months) |
7 |
| Ozutemiz 2017 [73] |
64(M) |
Lateral ventricle |
S |
|
NS |
273 |
| Mascarenhas 2017 [74] |
35(F) |
Prepontine |
S |
|
NS |
NS |
| Roh 2017 [21] |
53(F) |
CPA |
S +RT |
67.2 Gy |
Deceased (4 years) |
NS |
| Kwon 2019 [15] |
71(M) |
CPA |
S |
|
Alive (2 months) |
NS |
| Cuoco 2019 [20] |
71(M) |
CPA |
S + SRS |
25 Gy |
NS |
60 |
| Fereydonyan 2019[2] |
30(M) |
CPA |
S +RT |
NS |
Alive (2 years) |
60 |
| Demuth 2019 [75] |
67(F) |
CPA |
S + ChT |
|
Deceased (time NS) |
NS |
| Zuo 2021 [76] |
39(M) |
CPA |
S |
|
Deceased (4 months) |
NS |
| Zuo 2021 [76] |
54(F) |
Suprasellar |
S |
|
Deceased (4 months) |
NS |
| Zuo 2021 [76] |
43(M) |
CPA |
S + PB |
NS |
Alive (72 months) |
NS |
| Zuo 2021 [76] |
44(M) |
CPA |
S + RT |
NS |
Deceased (10 months) |
48 |
| Zuo 2021 [76] |
51(M) |
CPA |
S + RT |
NS |
Deceased (3 months) |
NS |
| Zuo 2021 [76] |
48(M) |
CPA |
S |
Deceased |
(9 months) |
180 |
| Zuo 2021 [76] |
61(M) |
CPA |
S + RT |
NS |
Deceased (23 months) |
336 |
| Zuo 2021 [76] |
61(M) |
CPA |
S |
|
Deceased (12 months) |
|
| Zuo 2021 [76] |
60(M) |
CPA |
S + SRS |
NS |
Deceased (13 months) |
60 |
Abbreviations: MT: Malignant Transformation; M: Male; F: Female; CPA: Cerebellopontine Angle; S: Surgery; RT: Radiation Therapy; SRS: Stereotactic
Radiosurgery; PB: Proton Beam; Cht: Chemotherapy; BSC: Best Supportive Care; NS: Not Specified.
Table 2: Reported cases of primary intracranial squamous cell carcinoma.
| Author, year |
Age (sex) |
Location |
Treatment |
Dose |
Survival status at last
evaluation
(time from
surgery)
|
| Wong 1976 [5] |
4(M) |
Parapontine |
Autopsy |
|
Deceased (1 day) |
| Nosaka 1979 [7] |
46(M) |
CPA |
S |
|
Deceased (7 months) |
| Garcia 1981 [18] |
61(M) |
CPA |
RT |
NS |
Deceased (9 months) |
| Ebisudani 1990 [8] |
68(M) |
CPA |
S |
|
Deceased (1 month) |
| Jain 2003 [9] |
5(F) |
Temporal lobe |
S + ChT + RT |
NS |
Alive (10 months) |
| Mallick 2012 [10] |
35(F) |
Frontal lobe |
S + RT |
60 Gy |
Alive (12 months) |
| O'Neill 2016 [11] |
49(M) |
Sellar |
S + SRS |
NS |
Alive (12 months) |
| Liu 2018 [12] |
20(M) |
Lateral ventricle |
S |
|
Alive (9 months) |
| Pisano 2020 [13] |
35(M) |
CPA |
S |
|
Deceased (1 month) |
| Mula-Hussain 2021 [14] |
NS(M) |
CPA |
S + RT + SRS |
RT: 54 Gy
SRS: 20 Gy
|
Alive (42 months) |
Garcia-Exposito 2023
(present
case report)
|
42(F) |
CPA |
S + RT |
50 Gy |
Deceased (26 months) |
Abbreviations: M: male; F: female; CPA: cerebellopontine angle; S: surgery; RT: radiation therapy; SRS: stereotactic radiosurgery; ChT: chemotherapy; NS: not specified.
Diagnostic criteria
In 1982, Garcia et al. [18] proposed the following diagnostic
criteria for primary intracranial SCC: Tumour limited to the intracranial, intradural compartment without extension beyond the
dura, cranial orifices, or connection with the middle ear, air sinuses, or sella turcica. Nasopharyngeal cancer must also be ruled
out. According to those authors, only cases that meet all of these
criteria should be considered primary intracranial SCC. In their
study, Hamlat and colleagues [16] included two other diagnostic
criteria: the presence of a benign squamous cell epithelium within
the malignant tumour and the absence of metastatic carcinoma.
Radiological findings
The most commonly reported location of these lesions is the
CPA (45% of cases), followed by the temporal lobe (12%), and, less
commonly, the prepontine area and cerebellum [15,17].
Most lesions described to date have a low signal intensity on
T1-Weighted MRI Images (T1-WI) and high signal intensity on
T2-weighted images (T2WI) [4,20-22]. The presence of contrast
enhancement and oedema may be key findings suggestive of
malignant transformation [23]. Nawashiro et al. [24] found that
epidermoid cysts could be differentiated from malignant tumours
on Diffusion-Weighted Imaging (DWI), with a high signal on DWI
in benign cysts versus a low signal in malignant transformations.
Leptomeningeal carcinomatosis, which is associated with a
poor prognosis, has been reported in 27.8% of patients [4]. The
development of LC may be due to leptomeningeal seeding by tumour cells or wide dissemination of malignant cells from an intradural primary lesion. Given that leptomeningeal metastases have
been described in locations far from the primary lesion and without clear continuity, some authors have suggested that LC could
also be due to surgical dissemination [25].
Treatment
In benign intracranial epidermoid lesions, the initial approach
is usually surgery. By contrast, no standard approach has yet been
developed for SCC. Liu et al. [4] categorized the treatment options
into several groups, as follows: surgical management alone; surgery plus radiotherapy (either radiosurgery or stereotactic radiosurgery); chemotherapy alone; or chemotherapy in combination
with either surgery or radiotherapy; or surgery plus one or more
adjuvant therapies.
Surgery
Surgically-treated patients appear to have a significantly better
prognosis than patents who receive palliative treatments (median
survival: 25 vs. 8 months); moreover, gross total resection is associated with better survival outcomes than subtotal resections
(median survival: 48 vs. 35 months, respectively) [15]. The addition of adjuvant radiotherapy to primary surgery is also associated
with better survival than surgery alone (35 vs. 5 months, respectively) [15,17].
Radiation therapy
In the patients included in the studies in this review (Table
1), the mean radiation dose was 52.0 Gy (range, 36.0-67.2 Gy);
however, higher doses were not associated with better survival
outcomes [15,17]. Moreover, according to Alvord’s model [26], a
2 cm epidermoid tumour has a 90% probability of growth inhibition when the total dose is 50 Gy (2 Gy daily fractions), without
an increased risk of radionecrosis or optic or neurocognitive impairment.
Tamura et al. [22] found that the addition of either conventional radiotherapy or radiosurgery to primary surgery positively
impacted survival outcomes. In that study, median survival times
for patients treated with surgery alone, surgery plus conventional radiotherapy, and surgery plus radiosurgery were 1,18, and 44
months, respectively.
Chemotherapy
Adjuvant chemotherapy may improve local control but its impact on survival is not clear due to the heterogeneous treatment
protocols applied to date and to the limited number of reported
cases in the literature.
Survival
Regardless of the specific treatment protocol, the reported recurrence rate is approximately 20%, with an average recurrence
time of 17 months [15,16]. Median survival following detection of
a recurrence is five months [15].
In patients with intracranial SCC, survival outcomes are poor,
and most patients die within 12 months following symptom onset
or diagnosis [16].
Conclusion
Primary intracranial squamous cell carcinomas are rare and the
optimal treatment approach remains unclear. The most common
approach appears to be surgery with adjuvant radiotherapy. Nevertheless, the probability of long-term survival in these patients
remains low, even in those who undergo gross total resection
with adjuvant radiotherapy.
In the present case, the presence of leptomeningeal progression (Figures 4 and 5) underscores the importance of neural axis
follow-up. Moreover, given that leptomeningeal dissemination
has been reported in close to 30% of patients, prophylactic irradiation of the entire neuroaxis should be considered as this treatment could potentially improve survival outcomes.
References
- Ernst P. Haufung dysontogenetischer Bildungen am Zentralnervensystem. Verb Dtsch Pathol Ges. 1912; 15: 226-230.
- Fereydonyan N, Taheri M, Kazemi F. Cerebellar Squamous Cell Carcinoma Due to Malignant Transformation of Cerebellopontine Angle Epidermoid Cyst, Report an Interesting Case and Review the Literature. Prague Medical Report. 2019; 120: 95-102.
- Lewis A, Cooper P, Kassel E, et al. Squamous cell carcinoma arising in a suprasellar epidermoid cyst. Journal of Neurosurgery. 1983; 59: 538-541.
- Liu X, Chen Z, Dong Y, et al. Primary Intracranial Squamous Cell Carcinoma Arising De Novo: A Case Report and Review of the Literature. World Neurosurgery. 2018; 120: 372-381.
- Wong S, Ducker T, Powers J. Fulminating parapontine epidermoid carcinoma in a four-year-old boy. Cancer. 1976; 37: 1525-1531.
- Lakhdar F, Hakkou E, Gana et al. Malignant Transformation Six Months after Removal of Intracranial Epidermoid Cyst: A Case Report. Case Reports in Neurological Medicine. 2011; 2011: 1-4.
- Nosaka Y, Nagao S, Tabuchi K. et al. Primary intracranial epidermoid carcinoma. Journal of Neurosurgery. 1979; 50: 830-833.
- Ebisudani D, Hamazaki F, Oka H, et al. An autopsy case of primary intracranial squamous cell carcinoma. Neurological surgery. 1990; 18: 193-198.
- Jain R, Gujar S, McKeever P, et al. Imaging findings associated with childhood primary intracranial squamous cell carcinoma. AJNR. American journal of neuroradiology. 2003; 24: 109-111.
- Mallick S, Biswas A, Kumar et al. Primary intracranial basaloid squamous cell carcinoma: An enigma. Neurologia i Neurochirurgia Polska. 2012; 46: 489-495.
- O’Neill B, Segkos K, Kasper E, et al. Non-metastatic squamous cell carcinoma within a Rathke’s cleft cyst. Pituitary. 2015; 19: 105-109.
- Liu X, Chen Z, Dong Y, et al. Primary Intracranial Squamous Cell Carcinoma Arising De Novo: A Case Report and Review of the Literature. World Neurosurgery. 2018; 120: 372-381.
- Pisano P, Lombardi F, Bongetta D, et al. Primary intracranial squamous cell carcinoma with a fatal course. Asian Journal of Neurosurgery. 2020; 15: 722.
- Mula-Hussain L, Malone J, dos Santos M, et al. Surgery and doseescalated radiotherapy for a de novo intracranial squamous cell carcinoma of the cerebellopontine angle. Clinical and Translational Radiation Oncology. 2021; 27: 99-102.
- Kwon S, Kim J, Kim Y, et al. Treatment and Survival Outcomes of Primary Intracranial Squamous Cell Carcinoma. World Neurosurgery. 2019; 125: e1-e9.
- Hamlat A, Hua Z, Saikali S, et al. Malignant Transformation of Intra-Cranial Epithelial Cysts: Systematic Article Review. Journal of Neuro-Oncology. 2005; 74: 187-194.
- Nagasawa D, Yew A, Spasic M, Choy W, Gopen Q, et al. Survival outcomes for radiotherapy treatment of epidermoid tumors with malignant transformation. Journal of Clinical Neuroscience. 2012; 19: 21-26.
- Garcia C, McGarry P, Rodriguez F. Primary intracranial squamous cell carcinoma of the right cerebellopontine angle. Journal of Neurosurgery. 1981; 54: 824-828.
- Nagasawa D, Choy W, Spasic M, Yew A, Trang A, et al. An analysis of intracranial epidermoid tumors with malignant transformation: treatment and outcomes. Clinical Neurology and Neurosurgery. 2012; 115: 1071-1078.
- Cuoco J, Rogers C, Busch C, Apfel L, Entwistle J, et al. Intracranial Squamous Cell Carcinoma Arising From a Cerebellopontine Angle Epidermoid Cyst Remnant Four Decades After Partial Resection. Frontiers in Oncology. 2019; 9.
- Roh T, Park Y, Park Y, Kim S, Chang J. Intracranial squamous cell carcinoma arising in a cerebellopontine angle epidermoid cyst. Medicine. 2017; 96: e9423.
- Tamura K, Aoyagi M, Wakimoto H, Tamaki M, Yamamoto K, et al. Malignant transformation eight years after removal of a benign epidermoid cyst: A case report. Journal of Neuro-Oncology. 2006; 79: 67-72.
- Pikis S, and Margolin, E, 2016. Malignant transformation of a residual cerebellopontine angle epidermoid cyst. Journal of Clinical Neuroscience. 2016; 33: 59-62.
- Nawashiro H, Higo R, Tokumaru A, Tsuzuki N, Shima K. Diffusionweighted MRI of an intracranial epidermoid with malignant transformation. Neuroradiology. 2001; 43: 891-891.
- Kano T, Ikota H, Kobayashi S, Iwasa S, Kurosaki S, et al. Malignant transformation of an intracranial large epidermoid cyst with leptomeningeal carcinomatosis -case report-. Neurologia medicochirurgica. 2010; 50: 349-353.
- Alvord E. Growth rates of epidermoid tumors. Annals of Neurology. 1977; 2: 367-370.
- Hug O. Krebsbildung aus einem pialen Epidermoid. Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin. 1942; 308: 679-689.
- Yamanaka A, Hinohara S, Hashimoto T. Primary diffuse carcinomatosis of the spinal meninges accompanied with cancerous epidermal cyst of the base of the brain; report of a case of autopsy. Gan. 1955; 46: 274-276.
- Davidson S, Small J. Malignant Change in an Intracranial Epidermoid. Journal of Neurology, Neurosurgery & Psychiatry. 1960; 23: 176-178.
- Landers J, Danielski J. Malignant intracranial epidermoid cyst. Report of a case with leptomeningeal spread. Archives of Pathology & Laboratory Medicine. 1960; 70: 419-423.
- Komjatszegi S. Primary Intracranial Epidermoid Carcinoma. Journal of Neurosurgery. 1980; 52.
- Toglia J, Netsky M, Alexander E. Epithelial (Epidermoid) Tumors of the Cranium. Journal of Neurosurgery. 1965; 23: 384-393.
- Fox H, South E. Squamous cell carcinoma developing in an intracranial epidermoid cyst (cholesteatoma). Journal of Neurology, Neurosurgery & Psychiatry. 1965; 28: 276-281.
- Dubois P, Sage M, Luther J, et al. Malignant Change in an Intracranial Epidermoid Cyst. Journal of Computer Assisted Tomography. 1981; 5: 433-435.
- Takado M, Hirose Genjiro, et al. An autopsy case of primary parapontine epidermoid carcinoma. Rinshō shinkeigaku = Clinical neurology. 1982; 22: 579-85.
- Lewis A, Cooper P, Kassel E, et al. Squamous cell carcinoma arising in a suprasellar epidermoid cyst. Journal of Neurosurgery. 1983; 59: 538-541.
- Giangaspero F, Manetto V, Ferracini R, et al. Squamous cell carcinoma of the brain with sarcoma-like stroma. Virchows Archiv A Pathological Anatomy and Histopathology. 1984; 402: 459-464.
- Maffazzoni D, Barbosa-Coutinho L, Chemalle I, et al. Carcinoma originado em cisto epidermoide intracraniano: registro de caso. Arquivos de Neuro-Psiquiatria. 1986; 44: 391-394.
- Kubokura T, Nishimura T, Tsubone K. Ct Scan Findings of Malignant Epidermoid Cyst. Neurologia Medico-chirurgica. 1986; 26: 706-711.
- Goldman S, Gandy S. Squamous cell carcinoma as a late complication of intracerebroventricular epidermoid cyst. Journal of Neurosurgery. 1987; 66: 618-620.
- Salazar J, Vaquero J, Saucedo G, Bravo G. Posterior fossa epidermoid cysts. Acta Neurochirurgica. 1987; 85: 34-39.
- Abramson R, Morawetz R, Schlitt M. Multiple Complications from an Intracranial Epidermoid Cyst: Case Report and Literature Review. Neurosurgery. 1989; 24: 574-578.
- Nishiura I, Koyama T, Handa J, Amano S. Primary Intracranial Epidermoid Carcinoma. Neurologia Medico-chirurgica. 1989; 29: 600-605.
- Gi H, Yoshizumi H, Nagao S, Nishioka T, Uno J, et al. C-P angle epidermoid carcinoma: A case report. No shinkei geka. Neurological surgery. 1990; 18: 1041-1045.
- Tognetti F, Lanzino G, Manetto V, Calbucci F. Intracranial squamous cell carcinoma arising in remnant of extirpated epidermoid cyst. British Journal of Neurosurgery. 1991; 5: 303-305.
- Acciarri N, Padovani R, Foschini M, Giulioni M, Finizio F. Intracranial squamous cell carcinoma arising in an epidermoid cyst. British Journal of Neurosurgery. 1993; 7: 565-569.
- Nishio S, Takeshita I, Morioka T, Fukui M. Primary Intracranial Squamous Cell Carcinomas. Neurosurgery. 1995; 37: 329-332.
- Uchino A, Hasuo K, Matsumoto S, Uda K, Moriguchi M, et al. Intracranial epidermoid carcinoma: CT and MRI. Neuroradiology. 1995; 37: 155-158.
- Ogata N, Jochum W, Aguzzi A, Fournier J, Yonekawa Y. Total removal of a primary intracranial squamous cell carcinoma invading the brain stem. Surgical Neurology. 1996; 46: 477-480.
- Mohanty A, Kolluri V, Santosh V. Squamous cell carcinomatous change in a posterior fossa epidermoid: Case report with a review of the literature. British Journal of Neurosurgery. 1996; 10: 493-496.
- Bayindir C, Balak N, Karasu A. Micro-invasive squamous cell carcinoma arising in a pre-existing intraventricular epidermoid cyst. Acta Neurochirurgica. 1996; 138: 1008-1012.
- Murase S, Yamakawa H, Ohkuma A, Sumi Y, Kajiwara M, et al. Primary Intracranial Squamous Cell Carcinoma. Neurologia Medicochirurgica. 1999; 39: 49-54.
- Asahi T, Kurimoto M, Endo S, Monma F, Ohi M, et al. Malignant transformation of cerebello-pontine angle epidermoid. Journal of Clinical Neuroscience. 2001; 8: 572-574.
- Khan R, Giri D, Rosenblum M, Petito F, De Angelis L. Leptomeningeal metastasis from an intracranial epidermoid cyst. Neurology. 2001; 56: 1419-1420.
- Link M, Cohen P, Breneman J, Tew J. Malignant squamous degeneration of a cerebellopontine angle epidermoid tumor. Journal of Neurosurgery. 2002; 97: 1237-1243.
- Monaco R, Boscaino A, Di Blasi A, D’Antonio A, Profeta G, et al. Intraepithelial carcinoma arising in an endodermal cyst of the posterior fossa. Neuropathology. 2003; 23: 219-224.
- Shirabe T, Fukuoka K, Watanabe A, Imamura K, Ishii R. Primary squamous cell carcinoma of the brain. A rare autopsy case. Neuropathology. 2003; 23: 225-229.
- Hamlat A, Hua Z, Saikali S, Egretau J, Guegan Y. Malignant transformation of intracranial epidermoid cyst with leptomeningeal carcinomatosis: case report. Acta Neurologica Belgica. 2003; 103: 225-229.
- Park JW, Park YM. Primary intracranial epidermoid carcinoma. J Korean Neurosurg Soc. 2003; 34: 159-161.
- Michael L, Moss T, Madhu T, Coakham H. Malignant transformation of posterior fossa epidermoid cyst. British Journal of Neurosurgery. 2005; 19: 505-510.
- Kodama H, Maeda M, Hirokawa Y, Suzuki H, Hori K, et al. MRI findings of malignant transformation of epidermoid cyst: case report. Journal of Neuro-Oncology. 2006; 82: 171-174.
- Pagni F, Brenna A, Leone B, Vergani F, Isimbaldi G. Malignant epidermoid cyst of the pineal region with lumbar metastasis. Neuropathology. 2007; 27: 566-569.
- Agarwal S, Rishi A, Suri V, Sharma M, Satyarthi G, et al. Primary intracranial squamous cell carcinoma arising in an epidermoid cyst-A case report and review of literature. Clinical Neurology and Neurosurgery. 2007; 109: 888-891.
- Kim M, Kim O. Primary Intracranial Squamous Cell Carcinoma in the Brain Stem with a Cerebellopontine Angle Epidermoid Cyst. Journal of Korean Neurosurgical Society. 2008; 44: 401.
- Ge P, Luo Y, Fu S, et al. Recurrent Epidermoid Cyst With Malignant Transformation Into Squamous Cell Carcinoma. Neurologia Medico-chirurgica. 2009; 49: 442-444.
- Hao S, Tang J, Wu Z, et al. Natural Malignant Transformation of an Intracranial Epidermoid Cyst. Journal of the Formosan Medical Association. 2010; 109: 390-396.
- Nakao Y, Nonaka S, Yamamoto T, et al. Malignant Transformation 20 Years after Partial Removal Of Intracranial Epidermoid Cyst. Neurologia Medico-chirurgica. 2010; 50: 236-239.
- Chon K, Lee J, Koh E, et al. Malignant Transformation of an Epidermoid Cyst in the Cerebellopontine Angle. Journal of Korean Neurosurgical Society. 2012; 52: 148.
- Feng R, Gu X, Hu J, et al. Surgical treatment and radiotherapy of epidermoid cyst with malignant transformation in cerebellopontine angle. International Journal of Clinical and Experimental Medicine. 2014; 7: 312-315.
- Raheja A, Eli I, Bowers C, et al. Primary Intracranial Epidermoid Carcinoma with Diffuse Leptomeningeal Carcinomatosis: Report of Two Cases. World Neurosurgery. 2016; 88: 692.e9-692.e16.
- Solanki S, Maccormac O, Dow G, et al. Malignant transformation of residual posterior fossa epidermoid cyst to squamous cell carcinoma. British Journal of Neurosurgery. 2016; 31: 497-498.
- Ding S, Jin Y, Jiang J. Malignant Transformation of an Epidermoid Cyst in the Temporal and Prepontine Region: Report of a Case and Differential Diagnosis. Oncology Letters. 2016; 11: 3097-3100.
- Ozutemiz C, Ada E, Ersen A, et al. Imaging findings of an epidermoid cyst with malignant transformation to squamous cell carcinoma. Turkish Neurosurgery. 2014.
- Mascarenhas A, Parsons A, Smith C, et al. Malignant squamous cell carcinoma arising in a previously resected cerebellopontine angle epidermoid. Surgical Neurology International. 2017; 8: 186.
- Demuth S, Lasry D, Obaid S, et al. Pseudo-Chemical Meningitis and the Malignant Transformation of an Epidermoid Cyst. Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques. 2019; 46: 642-644.
- Zuo P, Sun T, Wang Y, et al. Primary Squamous Cell Carcinomas Arising in Intracranial Epidermoid Cysts: A Series of Nine Cases and Systematic Review. Frontiers in Oncology. 2021; 11