Introduction
The therapeutic landscape for metastatic melanoma is rapidly changing with novel immunotherapy agents, which have demonstrated significantly improved response rates and outcomes
compared with conventional chemotherapy. Immune Checkpoint
Inhibitor (ICI) options for stage IV melanoma include anti-PD-1
monotherapy with pembrolizumab or nivolumab or nivolumab/
ipilimumab combination therapy [1-6]. Ipilimumab is a CTLA-4
inhibitor, and CTLA-4/PD-1 inhibitor combination therapies from
the Checkmate 067 and Checkmate 069 results demonstrated a
higher response rate with nivolumab/ipilimumab combination
therapy compared with ipilimumab alone [3-5,7,8].
Cancer-related inflammation responses, such as increased and
defective myelopoiesis and both local and systemic inflammation,
play an important role in tumorigenesis, disease progression, and
patient prognosis [9-11]. Several studies have demonstrated the
Neutrophil to Lymphocyte Ratio (NLR) to be associated with clinical outcomes regardless of cancer type [12-14].
Platelets are also well-known as an important indicator of systemic inflammation [15] by secreting various cytokines to support
tumor growth, protecting tumors from apoptosis, and promoting
tumor metastasis [16]. The prognostic and predictive roles of platelets and the Platelet to Lymphocyte Ratio (PLR) in melanomas
have been less investigated, and published data remain controversial [17,18].
In this study, we evaluated the associations of several blood
counts and their ratios at baseline with prognostic factors, Overall
Survival (OS), and Progression-Free Survival (PFS) in patients with
metastatic melanoma treated with first-line ICI.
Materials and methods
Data collection
Among the patients with metastatic melanoma who received
immune checkpoint inhibitors (nivolumab or pembrolizumab) as
a first-line treatment at Samsung Medical Center between December 2016 and June 2021, 82 with available hematological values before initiating ICIs were included in the analysis. For all patients, White Blood Cell (WBC) count, Absolute Neutrophil Count
(ANC), Absolute Lymphocyte Count (ALC), platelet count within
2 weeks prior to initiation of therapy, and other clinical and molecular data were collected before beginning ICI treatment. NLR
was calculated as ANC/ALC, derived NLR (dNLR) was calculated
as ANC/ (WBC-ALC), and PLR was calculated as platelet/ALC. This
study was approved by the Institutional Review Board of Samsung
Medical Center. The requirement of informed consent was waived
due to the retrospective nature of this study.
Response evaluation
Tumor assessment was performed at baseline, 6-9 weeks, and
every 8-9 weeks thereafter, and clinical responses were classified
according to the response evaluation criteria in solid tumors (RECIST 1.1). Response Rate (RR) was calculated as the percentage
of patients experiencing a confirmed Complete Response (CR) or
Partial Response (PR), and Disease Control Rate (DCR) was calculated as RR + Stable Disease (SD) per the RECIST 1.1 guidelines.
Statistical analysis
Progression-Free Survival (PFS) was determined from the first
cycle of treatment to disease progression documented by imaging, death (event), or last follow-up (censored). Overall Survival
(OS) was calculated from the first cycle of treatment to death
(event) or last follow-up (censored). Data cut-off for survival
analysis was set at May 30, 2023. Pearson’s chi-square test or
Fisher’s exact test was used to compare discrete data. Continuous
variables were transformed into categorical variables by X-Tile
software (Yale University, New Haven, CT, USA) and determined
the best cut-off values for each parameter [19]. Survival outcomes
were estimated using the Kaplan-Meier method and compared
using the log-rank test. Multivariate analysis was performed by
Cox-regression analysis. The analyses were conducted using the
Statistical Package for the Social Sciences (SPSS), version 19.0
(SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
We collected data for 82 patients treated either with nivolumab or pembrolizumab. All patients were treated with ICIs as
first line treatment -68(82.9) patients with pembrolizumab, eight
(9.8%) with nivolumab, four (4.9%) with pembrolizumab +/- lenvatinib, and two (2.4%) patients with nivolumab plus ipilimumab.
Baseline patient characteristics are presented in Table 1. The median age was 60 years (range: 27-82 years), and the numbers of
females and males were 40(49%) and 42(51%), respectively. The
most common site of primary cancer was cutaneous melanoma
(N=67, 61%), followed by mucosal type (N=35, 42.7%), uveal
melanoma (N=3, 3.7%), and acral melanoma (N=2, 2.4%). BRAF
V600E-mutated tumors were observed in 12 patients (14.6%).
Response and survival
Of the 82 patients, 13(15.9%) achieved Complete Response
(CR), 31 PR (37.8 %), 19 SD (23.2%), and 16 had progressive disease (PD) (23.2%) (Table 2). The Overall Response Rate (ORR)
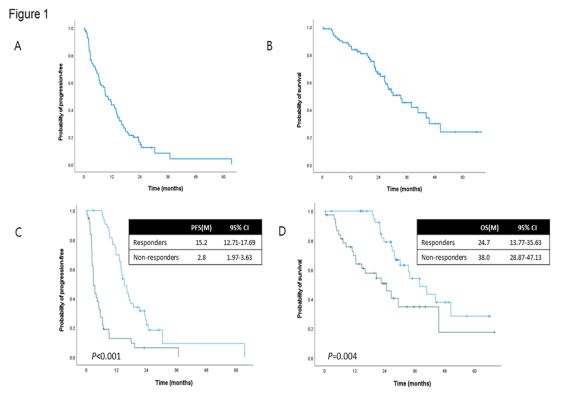
and disease control rate (DCR) were 53.7% and 76.9%, respectively. Among all patients, the median PFS was 10.3 months (95%
confidence interval (CI): 7.30-13.30) and the median OS was 33.5
months (95% CI 24.78-42.22) (Figure 1).
Forty-four patients were categorized as responders and 40
patients as non-responders. There was a significant difference in
median PFS between the groups: 15.2 months [95% Confidence
Interval (CI), 12.71-17.69] in the responders and 2.8 months
(95% CI, 1.97-3.63) in the non-responders [Hazard ratio (HR) 3.87
(95% CI, 2.36-3.63); P<0.001; Figure 1A]. The median OS was 38.0
months (95% CI, 28.87-47.13) in the responder group and 24.7
months (95% CI, 13.77-35.63) in the non-responder group [HR
2.43 (95% CI, 1.29-4.57); P=0.006; Figure 1B].
Blood counts
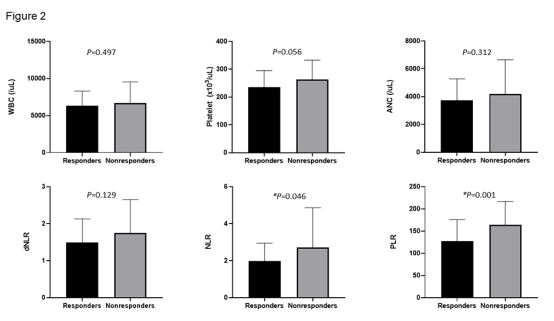
Among the various blood counts, mean levels of baseline NLR
and PLR were significantly lower in the responder group compared to the non-responder group (Figure 2). X-tile software was
used to verify the cut-off values in relation to PFS and OS. Regarding the total WBC and neutrophil counts, respective cut-off
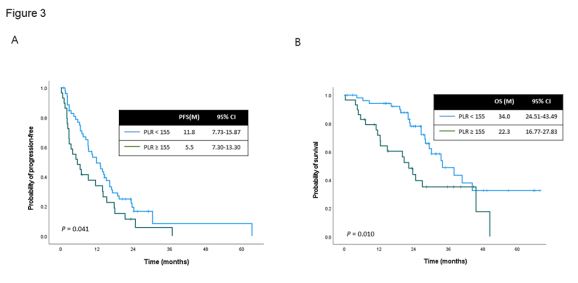
values were 5,180/uL and 3,100/uL. For platelets and PLR, cut-off values were 283,000/uL and 155, respectively. Univariable analyses of PFS and OS were performed for all patients, and the results are included in Table 3. Multivariable analysis identified high
serum platelets (≥283,000/uL) and high PLR (≥155) as significant
prognostic factors for worse PFS, and high PLR was identified as an
independent prognostic factor for worse OS. Median PFS of 11.8
months (95% CI, 7.73-15.87) was observed in patients with low
PLR and of 5.5 months (95% CI, 7.30-13.30) in patients with high
PLR (P=0.041; Figure 3A). Median OS was significantly prolonged
up to 34.0 months (95% CI, 24.51-43.49) in patients with low PLR
and to 22.3 months (95% CI, 16.77-27.83) in patients with high
PLR (P=0.010; Figure 3B).
Table 1: Baseline characteristics.
|
No (%) |
| Total N |
82 (100) |
|
Mean age +/- SD (range),
years
|
60 +/- 12.18 (27-82) |
| Sex |
|
| Male |
42 (51.2) |
| Female |
40 (48.8) |
| ECOG PS |
|
| 0 |
24 (29.2%) |
| 1 |
58 (70.7%) |
| Subtype |
|
| Acral |
2 (2.4) |
| Cutaneous |
42 (51.2) |
| Mucosal |
35 (42.7) |
| Uveal |
3 (3.7) |
| BRAF V600 status |
|
| Mutant |
12 (14.6) |
| Wild type |
70 (85.4) |
| First-line treatment |
|
| Nivolumab |
8 (9.8) |
| Nivolumab + Ipilimumab |
2 (2.4) |
| Pembrolizumab |
68 (82.9) |
|
Pembrolizumab +/- Lenvatimib
|
4 (4.9) |
| Baseline CBC |
|
| Mean WBC +/- SD (range) |
6,513 +/- 263.61
(3,160-17,970)
|
| Mean ANC +/- SD (range) |
3,935 +/- 224.11
(1,490-15,200)
|
| Mean ALC +/- SD (range) |
1,911 +/- 77.82 (680-4,400)
|
|
Mean Platelet +/- SD (range)
|
248,270 +/- 274.4
(122,000-400,000)
|
| Mean NLR +/- SD (range) |
2.3 +/- 0.18(0.7-13.7) |
|
Median PLR +/- SD (range)
|
144.2 +/- 5.95 (60.5-283.8)
|
Abbreviations: ICI: Immune checkpoint inhibitor; WBC: White blood
cell count; ANC: Absolute neutrophil count; ALC: Absolute lymphocyte
count; NLR: Neutrophil to lymphocyte ratio; PLR: Platelet to lymphocyte
ratio.
Table 2: Clinical activity of first-line immune checkpoint inhibitors.
| Response |
First-line immune checkpoint
inhibitor (N=82)
|
| No. |
% |
| Complete response |
13 |
15.9 |
| Partial response |
31 |
37.8 |
| Stable disease |
19 |
23.2 |
| Progressive disease |
19 |
23.2 |
| Objective response rate |
44 |
53.7 |
| Disease control rate |
63 |
76.8 |
Table 3: Univariable analysis for prognostic factors.
|
PFS |
OS |
| HR |
95% CI |
P value |
HR |
95% CI |
P value |
| Age |
|
|
|
|
|
|
| ≥65 versus <65 (Ref) |
0.95 |
0.58-1.54 |
0.822 |
1.52 |
0.81-2.86 |
0.194 |
| Sex |
|
|
|
|
|
|
|
Male versus Female (Ref)
|
1.11 |
0.68-1.79 |
0.683 |
0.87 |
0.46-1.62 |
0.650 |
| BRAF mutation |
|
|
|
|
|
|
| Yes versus No (Ref) |
0.96 |
0.47-1.94 |
0.900 |
0.34 |
0.08-1.43 |
0.142 |
| TMB |
|
|
|
|
|
|
| High versus Low (Ref) |
0.49 |
0.20-1.23 |
0.127 |
0.37 |
0.09-1.55 |
0.174 |
| Serum WBC (/uL) |
|
|
|
|
|
|
|
≥5,180 versus <5,180
(Ref)
|
0.61 |
0.36-1.03 |
0.066 |
0.68 |
0.36-1.32 |
0.255 |
| Serum ANC |
|
|
|
|
|
|
|
≥3,100 versus <3,100
(Ref)
|
0.65 |
0.39-1.10 |
0.108 |
0.86 |
0.45-1.64 |
0.635 |
|
Serum platelet (x103/uL)
|
|
|
|
|
|
|
|
≥283 versus <283 (Ref)
|
1.71 |
1.02-2.88 |
0.043 |
0.79 |
0.38-1.62 |
0.513 |
| dNLR |
|
|
|
|
|
|
|
≥2.06 versus <2.06 (Ref)
|
0.66 |
0.37-1.20 |
0.176 |
1.27 |
0.60-2.68 |
0.538 |
| NLR |
|
|
|
|
|
|
|
≥2.0 versus <2.0 (Ref)
|
1.27 |
0.78-2.07 |
0.346 |
1.27 |
0.67-2.44 |
0.464 |
| PLR |
|
|
|
|
|
|
|
≥155 versus <155 (Ref)
|
1.65 |
1.01-2.68 |
0.044 |
2.23 |
1.20-4.16 |
0.012 |
Abbreviations: PFS: Progression-free survival; OS: Overall survival; HR: Hazard ratio; CI: Confidence
interval; Ref: Reference; TMB: Tumor mutation burden; WBC: White blood cell count; ANC: Absolute
neutrophil count; dNLR: derived neutrophil to lymphocyte ratio; NLR: Neutrophil to lymphocyte ratio;
PLR: Platelet to lymphocyte ratio.
Discussion
We described the clinical outcomes of first-line ICI in 82 Korean patients with metastatic melanoma and potential blood
biomarkers to predict the response to ICI at a single institution.
The acral/mucosal, uveal, and cutaneous subtypes accounted for
45.1%, 3.7%, and 51.2% of ICIs, respectively. The overall ORR was
53.7%, while ORRs for the acral/mucosal and cutaneous subtypes
were 56.8% and 54.8%, respectively. All three patients with uveal
melanoma did not achieve more than a partial response, and the
overall DCR of all patients was 76.9%.
Clinical responders to ICI treatment exhibited significantly better survival outcomes than non-responders. Responsiveness to
first-line ICI is considered to improve PFS and OS rates [20], as
observed in the present study where responders exhibited higher
PFS and OS rates compared to non-responders. Regarding the baseline various blood counts, mean NLR and PLR were significantly
lower in the responder group compared to the non-responder
group. Mean WBC, ANC, and platelet count were also lower in
the responder group than in the non-responder group, but the
difference was not significant.
In our retrospective cohort study, the median PFS of all patients was 10.3 months and the median OS was 33.5 months, comparable to those of phase 3 clinical trials [21,22]. We determined that higher platelet count and PLR value exhibited a negative impact on PFS, while higher PLR value closely correlated
with worse OS. There is no consensus regarding the optimal cutoff
value of PLR as a prognostic marker. Previous meta-analysis results identified a PLR cutoff of 120, and the pooled HRs of higher
PLR for OS and PFS in melanoma were 1.70 (95% CI, 1.22-2.37)
and 1.65 (95% CI, 1.10-2.47), respectively [18]. PLR ≥200 was associated with worse OS (HR: 1.94; 95% CI: 1.29-2.94; P=0.002) and
worse PFS (HR: 1.894; 95% CI: 1.27-2.82; P=0.002) in patients with
metastatic melanoma and non-small cell lung cancer on PD-1 inhibitors [23]. Patients with PLR ≥200 consisted of only 13.4% of
our cohort, and no significant difference was observed between patients with baseline PLR>200 or <200.
There were several studies that demonstrated elevated NLR as
a prognostic indicator for survival in melanoma patients treated
with PD-1 inhibitors [12,13,24]. In this study, the baseline mean
NLR value was significantly higher in non-responder patients.
However, NLR with multiple cut-offs did not exhibit a significant
association with PFS or OS. If there is a unified cut-off value for
NLR and PLR, it would be easy to predict the response to treatment for clinicians. However, since there is no such absolute value, further research in different cohorts is necessary.
This study had some limitations associated with its retrospective design, including the presence of uncontrolled confounding
factors, lack of Lactate Dehydrogenase (LDH) values, and absence
of information on adverse events. In addition, the sample size was
relatively small. However, we enrolled a homogeneous cohort of
patients with metastatic melanoma who received first-line ICI
treatment at a single institution, and we reported the predictive
role of PLR for first-line ICI treatment and also the prognostic value of PLR in metastatic melanoma in Korean patients.
Conclusion
In conclusion, our results demonstrated that high PLR above
155 was associated with poorer PFS and OS in patients with stage
IV melanoma treated with first-line immune checkpoint inhibitors.
Acknowledgments
Conflicts of interest statement: All the authors declare no
conflicts of interest.
Funding: No funding.
References
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015; 372: 320-330.
- Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, et al. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: ThreeYear Follow-up of a Randomized Phase 3 Trial. JAMA Oncol. 2019; 5: 187-194.
- Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018; 19: 1480-1492.
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017; 377: 1345-1356.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015; 373: 23-34.
- Robert C, Ribas A, Schachter J, Arance A, Grob JJ, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019; 20: 1239-1251.
- Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016; 17: 1558-1568.
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015; 372: 2006-2017.
- Kim MR, Kim AS, Choi HI, Jung JH, Park JY, et al. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PLoS One. 2020; 15: e0236445.
- Zhang L, Li L, Liu J, Wang J, Fan Y, et al. Meta-analysis of multiple hematological biomarkers as prognostic predictors of survival in bladder cancer. Medicine (Baltimore). 2020; 99: e20920.
- Qi Y, Liao D, Mei D, Zhang Y, Liu Y. Elevated Neutrophil-to-Lymphocyte Ratio Is Associated With Poor Outcomes for Melanoma Patients Treated With PD-1 Inhibitor or Chemotherapy in a Chinese Population. Front Oncol. 2020; 10: 1752.
- Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta Cruz JM, et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer. 2020; 126: 76-85.
- Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018; 6: 74.
- Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014; 106: dju124.
- Yamamoto T, Kawada K, Obama K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int J Mol Sci. 2021; 22: 8002.
- Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018; 11: 125.
- Han SN, Feng SJ, Liu Y. Prognostic and clinicopathological significance of the platelet-to-lymphocyte ratio in melanoma: A metaanalysis involving 2099 patients. Kaohsiung J Med Sci. 2021; 37: 55-62.
- Wang E, Huang H, Tang L, Tian L, Yang L, et al. Prognostic significance of platelet lymphocyte ratio in patients with melanoma: A meta-analysis. Medicine (Baltimore). 2021; 100: e27223.
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004; 10: 7252-7259.
- Lee J, Chang JS, Roh MR, Jung M, Lee CK, et al. Clinical Outcomes of Immune Checkpoint Blocker Therapy for Malignant Melanoma in Korean Patients: Potential Clinical Implications for a Combination Strategy Involving Radiotherapy. Cancer Res Treat. 2020; 52: 730-738.
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015; 16: 908-918.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019; 381: 1535-1546.
- Kartolo A, Holstead R, Khalid S, Emack J, Hopman W, et al. Serum neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in prognosticating immunotherapy efficacy. Immunotherapy. 2020; 12: 785-798.
- Wang C, Liu S, Li X, Cui K, Zhang W, et al. Baseline neutrophil-toratio combined with the change during treatment provides risk stratification for metastatic malignant melanoma patients treated with PD-1 inhibitors in a Chinese population. Front Oncol. 2023; 13: 1118301.