Introduction
EES belongs to the Ewing’s sarcoma family of tumors (ESFT).
ESFT shares a common aberrant fusion and is distinguished into
several subtypes based on cellular differentiation and anatomic
location, namely Ewing’s sarcoma of bone (ESB), EES, primitive
neuroectodermal tumor (PNET), and Askin tumor [1,2]. Compared to the second most common bone tumor, ESB, EES has a lower
incidence rate, a higher average age of onset, and is more likely
to arise in axial locations [3-5]. The paravertebral region is a relatively rare location for EES, and tumors almost arise from extradural paraspinal soft tissue in this region [6]. Therefore, IEES is
extremely rare and constitutes only 2.9% of all perispinal Ewing’s
sarcoma in adults [6,7]. What’s more, IEES with involvement of
the intervertebral foramen makes the case even rarer. In this article, we report an adult IEES in the lumbar spine and involving the
intervertebral foramen.
Case report
A 45-year-old gentleman presented with lower back pain in
early June 2022. The symptoms worsened after about half a month, when he experienced discomfort in the right lower extremity
(no lower extremity pain), and was seen at a local hospital. MRI
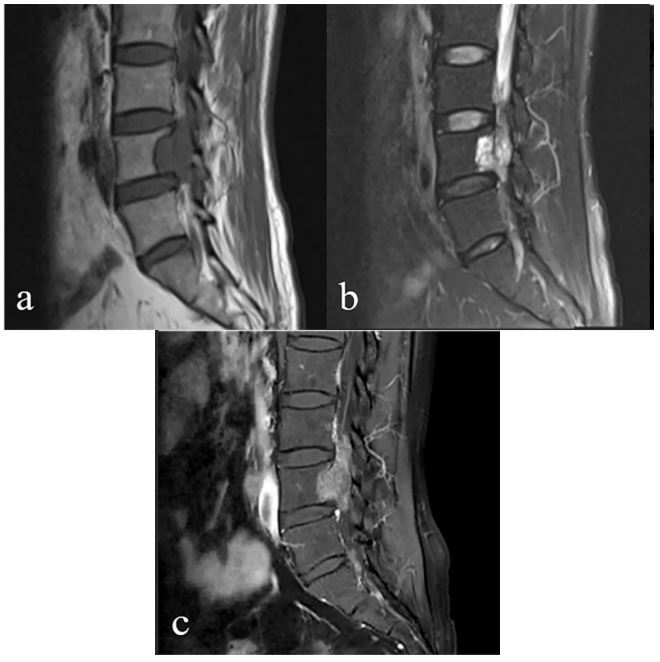
showed irregular clump-like shadow in the L4/5 right intervertebral foramen, about 26 mm × 37 mm × 44 mm, and it protruded into the spinal canal. T1-weighted imaging (T1WI) showed
equal signal, T2-weighted imaging (T2WI) and T2-weighted imaging with fat suppression (T2WI-FS) showed mixed hypersignal
shadow (Figure 1a-b). In early July of the same year, the patient
was admitted to our hospital with pain in his lower back and right
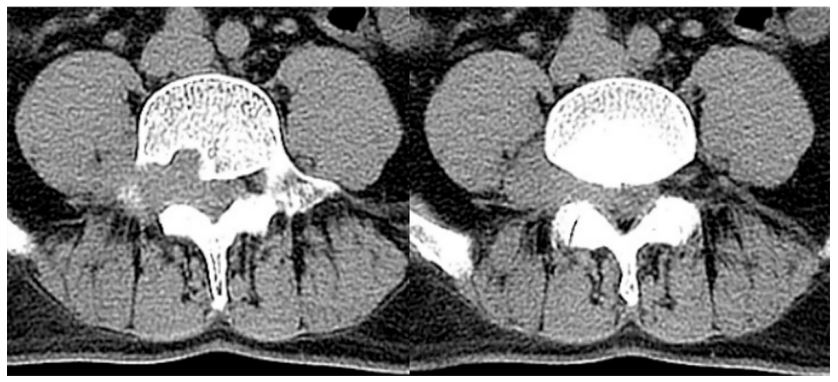
lower limb. Local bone resorption of the L4 vertebral body and
sclerotic edges can be seen on CT scan (Figure 2). No significant
abnormalities were seen on chest CT scan. Contrast-enhanced
MRI (CE-MRI) revealed a mass of about 45×42×23 mm in the spinal canal and right intervertebral foramen at the L4/5 level with
intense contrast enhancement (Figure 1c).
The patient underwent neuraxial tumor resection and internal
spinal fixation under general anesthesia. Under the positioning of
the C-arm machine, L3-4 laminas were disconnected and the L4-5
upper articular processes were excised. After longitudinal incision
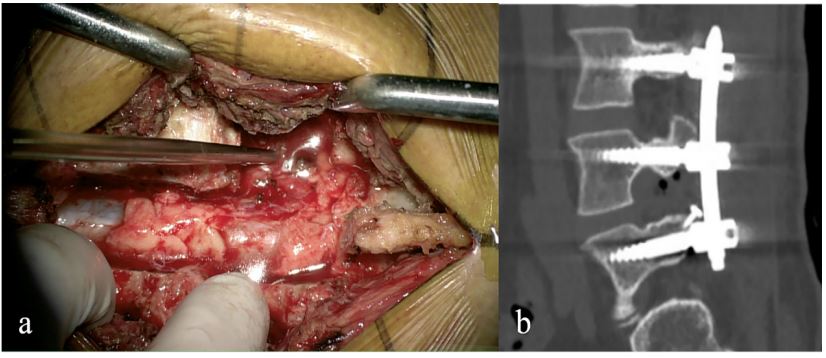
of the dura, the tumor was found located subdural and grew into
the L4-5 intervertebral foramen, about 4×4 cm with clear margin
and incomplete capsule (Figure 3a). The tumor was removed in
blocks under neuroelectrophysiological supervision, and the incision of the dural sac was sutured continuously. Connecting plates
were used to fix the repositioned L3-4 laminas. L3-5 were fixed
with pedicle screws, and the transverse processes were implanted
with bone mud and bone chips. An X-ray fluoroscopic machine
showed appropriate positions of the lumbar pedicle screws (Figure 3b), then the screw connecting rod was connected and locked. The drainage tube was inserted, the skin and subcutaneous
tissue were sutured layer by layer.
Immunohistochemistry revealed: CD99(+), FLI-1(+), Vim(+),
INI-1(+), S-100(partly+) Ki-67(+20%), CD56(partly+), Syn(-), CgA(-
) , CK(-), SOX-10(-), SMA(-), GFAP(-), CD45(-), Desmin(-), CD34(-),
WT-1(-), SSTR2(-), and H3K27M(-). Postoperative pathology of the
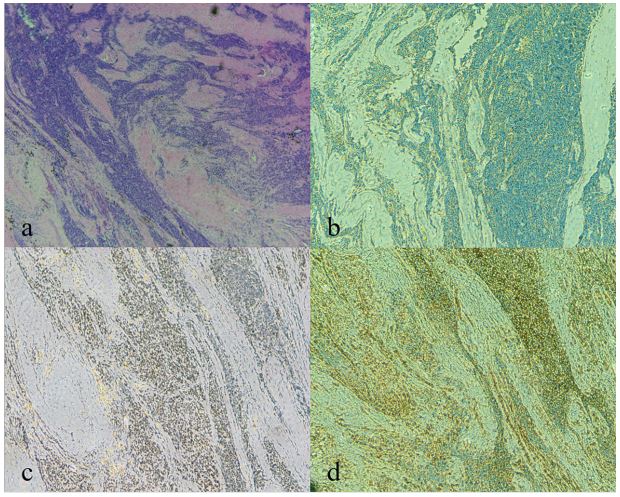
tumor tissue revealed small round cell malignancy, and the pathological diagnosis was EES (Figure 4). Molecular detection showed
positive for t(22q12) (EWSR1), confirming the diagnosis of Ewing’s
sarcoma.
The patient received 3 sessions of chemotherapy one and a
half months after surgery. The chemotherapy regimen is alternating VDC (vindesine, doxorubicin, and cyclophosphamide) and IE
(etoposide and ifosfamide), once a month. Seven months after
surgery, CE-MRI showed that the L3-5 vertebral body and adnexal internal fixation were in place, and there was no abnormal
strengthening in the spinal canal. The patient then continued to
receive chemotherapy 3 times with the same regimen as before.
Discussion
Reviewing the literature, nearly 50 cases of IEES has been reported from 1997 to 2022 [5-21]. IEES usually affects the lumbar/
sacral region as in our case [12]. The symptomatology is similar
with other space-occupying lesion of the spinal cord, influenced
by mass effect or invasion of adjacent structures, and the chief
complaint is pain as in our case [1,5,12,14,22]. Such clinical manifestations are difficult to distinguish from other intraspinal tumors
and may even be ignored by patient at early stage [6,10]. Thus,
diagnosis of these neoplasms is delayed. In our case, the symptom duration was at least one month, while the average duration
of the chief symptoms was 3 months [12].
Even if the imaging findings lack specificity, MRI is the preferred choice for tumor assessment in ESFT, including EES [1,10].
MRI reveals iso-signal intensity on T1WI, hypersignal intensity
corresponding to necrosis or cystic change on T2WI, and irregular
enhanced signaling after gadolinium administration [1,10,14]. Additionally, cortical erosion and periosteal reaction are seen in 40%
of cases of EES as in our case [1]. Chest CT are sensitive for detecting lung and other distant or nodal metastases [1]. However, in
previous literatures, masses were mostly seen only in the spinal
canal [5,6,10,12,22]. In our case, the mass was not only found in
the spinal canal, but also in one side of the intervertebral foramen.
The diagnosis of EES usually relies on postoperative pathological examination. On gross examination, the fish-like round mass
was seen at operation in our case, and it extended to the intervertebral foramen, invading the spinal cord and nerve roots. Prior to
our case, this rare manifestation of large-scale and intervertebral
foramen invasions had only been reported in very few IEES cases
[8]. Microscopically, the tumor shows large irregular sheets of
small round blue cells with scant clear or eosinophilic cytoplasm
and minimal extracellular matrix as in our case [2,10]. However,
small round blue cells also occur in other tumors like lymphoma
and rhabdomyosarcoma [1,10]. Therefore, further differentiation
is needed through immunohistochemistry.
A spectrum of immunohistochemical markers is used to study
EES [3]. Diffuse membranous expression of CD99 is characteristic for Ewing sarcoma (~95%) [2,5,23,24]. CD99 lacks specificity,
however, as it also expressed in other primitive neuroectodermal
tumors [23]. Friend leukemia integration 1 (FLI1) is highly expressed in cases of Ewing sarcoma with EWSR1-FLI1 gene fusion
and has relatively higher specificity than CD99 [2,3,23]. Besides,
vimentin (Vim) also diffusely expressed in these tumors [23]. The
patient in our case showed positive for CD99, FLI1, Vim, indicating
a favored diagnosis of EES.
At genetic level, reciprocal translocation t (11;22) (q24;q12) of
the EWSR1 gene on chromosome 22 with the FLI1 gene on chromosome 11 occurred in more than 85% of all ESFT cases [1,10,12].
Such translocation results in the EWSR1-FLI1 fusion gene acting
as an oncogenic transcription factor in ESFT [25]. The remaining
few cases contain fusions of the EWSR1 gene or FUS gene with
other ETS gene family members [26]. The patient in our case took
molecular detection and revealed positive for t(22q12) (EWSR1),
consistent with literature reports.
Treatment for spinal EES is difficult and currently not unified
due to the sparsity of literature and cases [10]. Surgical resection
is usually the initial intervention for EES in adults, especially for
the relief of spinal cord compression symptoms and cytoreductive purposes [25]. Due to the large extent of the lesion and the
involvement of intervertebral foramen, our patient underwent
posterior total laminectomy, took the screw-rod system fixation
and partly bone grafting. The internal fixation not only ensured
complete tumor removal, but also stabilized the spine and promoted bone graft fusion [27,28]. Chemotherapy regimens are
identical for EES and ESB, including vincristine, doxorubicin, and
cyclophosphamide alternating with ifosfamide and etoposide [1].
In our case, the patient received chemotherapy once a month
after surgical resection. Besides, radiotherapy is used in patients
with unresectable primary lesions, inadequate surgical margins,
or poor response to chemotherapy [1].
IEES has a high propensity for local recurrence and distant metastasis [14]. Relative studies showed a median progression free
survival (PFS) of 12 months and median overall survival (OS) of
14 months for spinal IEES [12]. In our case, the patient was reexamined 7 months after surgery and showed no obvious signs of
recurrent metastasis.
Conclusion
we provided an extremely rare case of adult IEES in the lumbar
spine and involving the intervertebral foramen. We also reviewed
relevant literature regarding its epidemiology, diagnosis, treatment and prognosis. Given the nonspecific clinical symptoms,
imaging manifestations, and highly progressive nature of spinal
EES, physicians should take the diagnosis of this type of tumor
into serious consideration, despite of their low incidence. Surgical
resection is still the initial intervention of the disease. We presented a surgical option that combined total laminectomy with pedicle screw internal fixation due to the large extent of the lesion.
Acknowledgements: The authors would like to acknowledge
and thank the individual patient involved who agreed to the publication of this article. And we also gratefully acknowledge the
Science Technology Department of Zhenjiang Province and Health
Science and Technology Plan of Zhejiang Province for their financial support.
Funding: This work has been supported by: Science Technology Department of Zhejiang Province (No. IGF22H160060), Health
Science and Technology Plan of Zhejiang Province (No. 2021KY794
and 2023KY147), and Wenzhou Municipal Key Laboratory of Neurodevelopmental Pathology and Physiology (2023HZSY0003).
References
- Wright A, Desai M, Bolan CW, et al. Extraskeletal Ewing Sarcoma from Head to Toe: Multimodality Imaging Review [J]. Radiographics : A review publication of the Radiological Society of North America, Inc. 2022; 42(4): 1145-60.
- Wei S, Siegal GP. Small Round Cell Tumors of Soft Tissue and Bone [J]. Arch Pathol Lab Med. 2022; 146(1): 47-59.
- Abboud A, Masrouha K, Saliba M, et al. Extraskeletal Ewing sarcoma: Diagnosis, management and prognosis [J]. Oncol Lett. 2021; 21(5): 354.
- Applebaum MA, Worch J, Matthay KK, et al. Clinical features and outcomes in patients with extraskeletal Ewing sarcoma [J]. Cancer. 2011; 117(13): 3027-32.
- zubuchi Y, Nakajima H, Honjoh K, et al. Primary intradural extramedullary Ewing sarcoma: A case report and literature review [J]. Oncol Lett. 2020; 20(3): 2347-55.
- Mungen E, Kurucu N, Kutluk T, et al. Primary spinal multifocal intradural-extramedullary Ewing sarcoma in children: presentation of a case and review of the literature [J]. Turk J Pediatr. 2021; 63(6): 1084-90.
- Yan Y, Xu T, Chen J, et al. Intraspinal Ewing’s sarcoma/primitive neuroectodermal tumors [J]. J Clin Neurosci. 2011; 18(5): 601-6.
- Ebrahimi R, Sohi A S M, Mirsardoo A, et al. Primary intradural extramedullary Ewing sarcoma in the lumbar area: A case report [J]. Radiol Case Rep. 2022; 17(12): 4617-21.
- Carballo Cuello C M, De Jesus O, De Jesus Espinosa A, et al. Prognosis and Outcome of Cervical Primary Extraosseous Intradural Extramedullary Ewing Sarcoma: A Systematic Review [J]. Cureus. 2022; 14(7): e26665.
- Pu F, Liu J, Zhang Z, et al. Primary intradural extramedullary extraosseous Ewing’s sarcoma/peripheral primitive neuroectodermal tumor (PIEES/PNET) of the thoracolumbar spine: A case report and literature review [J]. Open Med (Wars). 2021; 16(1): 1591-6.
- Karthigeyan M, Malik P, Sahoo S K, et al. Primary Spinal Intradural Ewing’s Sarcoma: Hemorrhagic Presentation with Acute Neurological Deterioration in Two Pediatric Patients [J]. Neurology India. 2021; 69(5): 1405-8.
- Lu V M, Goyal A, Alvi M A, Et Al. Primary intradural Ewing’s sarcoma of the spine: a systematic review of the literature [J]. Clinical neurology and neurosurgery. 2019; 177: 12-9.
- Takami H, Kumar R, Brown DA, et al. Histologic Features and Prognosis of Spinal Intradural Extramedullary Ewing Sarcoma: Case Report, Literature Review, and Analysis of Prognosis [J]. World neurosurgery. 2018; 115: 448-52.e2.
- Paterakis K, Brotis A, Tasiou A, et al. Intradural extramedullary Ewing’s sarcoma: A case report and review of the literature [J]. Neurologia i neurochirurgia polska. 2017; 51(1): 106-10.
- Bostelmann R, Leimert M, Steiger HJ, et al. The Importance of Surgery as Part of Multimodal Therapy in Rapid Progressive Primary Extraosseous Ewing Sarcoma of the Cervical Intra- and Epidural Space [J]. Clin Pract. 2016; 6(4): 897.
- Huh J, Kim KW, Park SJ, et al. Imaging Features of Primary Tumors and Metastatic Patterns of the Extraskeletal Ewing Sarcoma Family of Tumors in Adults: A 17-Year Experience at a Single Institution [J]. Korean J Radiol. 2015; 16(4): 783-90.
- Gong HS, Huang QS, Liu GJ, et al. Cervical Primary Ewing’s Sarcoma in Intradural and Extramedullary Location and Skip Metastasis to Cauda Equina [J]. Turk Neurosurg. 2015; 25(6): 943-7.
- Mardekian S K, Gandhe A, Miettinen M, et al. Two Cases of Spinal, Extraosseous, Intradural Ewing’s sarcoma/Peripheral Neuroectodermal Tumor: Radiologic, Pathologic, and Molecular Analysis [J]. Journal of clinical imaging science. 2014; 4: 6.
- Mateen F J, Nassar A, Bardia A, et al. Spinal intradural extraosseous Ewing’s sarcoma [J]. Rare Tumors. 2011; 3(1): 7.
- Karikari I O, Mehta A I, Nimjee S, et al. Primary intradural extraosseous Ewing sarcoma of the spine: case report and literature review [J]. Neurosurgery. 2011; 69(4): 995-9.
- Kim S W, Shin H. Primary Intradural Extraosseous Ewing’s Sarcoma [J]. Journal of Korean Neurosurgical Society. 2009; 45(3): 179-81.
- Xie N, Zhou Y. Clinical Reasoning: Longitudinally Extensive Spinal Cord Lesions in a Middle-aged Man [J]. Neurology. 2022; 98(10): 419-24.
- Muhuesein T M, Ilangovan G, Arul Pitchai ADP, et al. Extraskeletal Ewing’s Sarcoma With Vertebral Metastasis: A Case Report [J]. Cureus. 2022; 14(10): 30878.
- Gurria J P, Dasgupta R. Rhabdomyosarcoma and Extraosseous Ewing Sarcoma [J]. Children (Basel). 2018; 5(12).
- Bustoros M, Thomas C, Frenster J, et al. Adult Primary Spinal Epidural Extraosseous Ewing’s Sarcoma: A Case Report and Review of the Literature [J]. Case Rep Neurol Med. 2016; 2016: 1217428.
- Sbaraglia M, Righi A, Gambarotti M, et al. Ewing sarcoma and Ewing-like tumors [J]. Virchows Archiv : an international journal of pathology. 2020; 476(1): 109-19.
- Wang H, Huo Y, Li L, et al. Clinical Efficacy of Laminectomy with Instrumented Fixation in Treating Thoracolumbar Intradural Extramedullary Schwannomas: A Comparative Study [J]. Medical science monitor : international medical journal of experimental and clinical research. 2020; 26: 921719.
- Li H, Weng Y, Zhou D, et al. Experience of operative treatment in 27 patients with intraspinal neurilemmoma [J]. Oncol Lett. 2017; 14(4): 4817-21.