Introduction
Cancer treatment has evolved over the past twenty years with
the advent of immunotherapy and the development of cancer targeted therapies. Patients with cancer are living longer and often
with chronic pain related to their cancer. Subsequently, healthcare
clinicians are tasked with considering the adverse consequences
associated with their cancer treatment, including long-term
opioid therapy (LTOT). In a recently published study patients with
and without cancer had the same risk for adverse events, such
as overdose, from moderate to high dose opioids [1]. Increasing
evidence shows the use of LTOT itself carries adverse risks, including immune, endocrine, and mood dysfunction, as well as the
potential to trigger or develop NMOU and OUD. The prevalence of chronic non-malignant pain in the general population - for
which opioids are no longer recommended as first-line treatment
- is twenty percent [1]. A history of chronic non-malignant pain,
however, places patients at higher risk for severe pain requiring
opioids during cancer treatment [2]. Fifty-nine percent of patients
with cancer have pain [3], and cancer survivors continue to have a
higher utilization of LTOT than other US adults [4].
Opioids remain the standard of care for cancer pain treatment
[3] and evidence suggests that patients with cancer are at high
risk for SUD, or which we will use interchangeably with the term
NMOU [5,6]. NMOU is the use of an opioid for a non-pain symptom, differently from how it is prescribed and includes compulsive use of opioids, opioid use disorder (OUD) or other substances
(SUD). In this review, NMOU refers to the range of situations from
isolated instances of misuse to patterns of problematic use in
SUD. Management of NMOU can be challenging and may benefit
from a dedicated team approach [7]. Oncologists, who often are
primary opioid prescribers with their patients, can facilitate care
of patients who develop NMOU, by identifying when specialist
management is indicated. Optimal care utilizes an interdisciplinary team working towards a common outcome, including ongoing
engagement with cancer treatment and pain management to minimize adverse consequences on future health.
The Accreditation Council for Graduate Medical Education
(ACGME) has recognized the importance of these issues and
stated that oncology fellowships must include training on identifying NMOU and recognizing SUD [8].However, formal curricula
on these topics have not been published. Palliative care (PC) is
a valuable resource for oncologists seeking specialized pain and
symptom management, yet, data shows that PC clinicians have
insufficient training, lack of knowledge and low confidence in taking care of patients with SUD [9]. The minority of PC clinicians
who have specific primary addiction medicine training report improved competency and clinician satisfaction when taking care of
this patient population [10]. Similarly, PC clinicians who prescribe
in-office treatment of OUD with buprenorphine reported statistically significant increases in comfort and confidence managing
patients with NMOU and SUD compared to PC clinicians who only
received training without clinical practice change [11]. These studies suggest that comfort and confidence in managing patients
with NMOU and SUD can be improved by dedicated training and
practice support. Given the lack of training and confidence that
PC clinicians have with NMOU and LTOT despite recognition as
experts in cancer pain management, the barriers for oncologists
are even greater [10,12,13].
This article aims to describe the current state of knowledge in
the treatment of NMOU in people with cancer, empower oncology
teams to recognize the spectrum of intermittent NMOU to compulsive and problematic use consistent with SUD, and help oncology
clinicians understand potential management strategies. We will
incorporate a case-based format in this review of best practices.
Methods
In this narrative review, we utilized the IMRAD (Introduction,
Methods, Results, Discussion) Method described by Ferrari [14].
We queried PubMed and Google Scholar databases using combinations of the keywords: cancer, substance use disorder, addiction, non-medical opioid use, opioid use disorder, burnout, resiliency and education from the last 10 years (n=89,553). The
review was further limited to English language, clinical trials, randomized controlled trials, meta-analysis systematic review and
narrative reviews, with duplicates removed with total articles
included n=48 and additional references identified by a manual
search in the reference lists from retrieved articles, n=3 for a total
number of included articles of n=51. We present the content in a
case-based model.
Case 1
Chris is a 62-year-old with stage IV inoperable pancreatic cancer. They have a large pancreatic mass, as well as metastases in
the liver and lungs. Their current treatment is second line, leucovorin calcium (folinic acid), fluorouracil, irinotecan hydrochloride,
and oxaliplatin (FOLFIRINOX), and they have received 3 cycles.
When they were initially diagnosed with pancreatic cancer, abdominal pain was a significant complaint, and at that time they
were started on oxycodone 5 mg by mouth every 4 hours as needed. Their pain was initially uncontrolled on this regimen, and the
oxycodone was adjusted to 10 mg every 4 hours as needed. They
present today in follow up requesting a refill of oxycodone.
When you ask Chris how they are doing, they report they are
“doing well.” They deny pain, nausea, vomiting or constipation.
They report loose stools after their treatments, but these are
controlled with antidiarrheal medications. They report they do
not sleep well at night and use their oxycodone mostly at nighttime to help with sleep. Chris also reports that at times they feel
anxious and use their oxycodone as needed for their anxiety. They
are taking 1-2 oxycodone 10 mg per day.
Discussion: This case illustrates the nuance around opioid use
and why it is important to consistently ask how, when and why
patients are using their opioids. Chris is using their oxycodone for
sleep and anxiety and not pain which is defined as non-medical
opioid use (NMOU). Patients with cancer are at equal if not higher
risk of NMOU, with rates reported as 20% [15,16]. Patients with
cancer may have multiple other symptoms besides pain, in particular anxiety, insomnia or difficulty coping with their diagnosis
that may lead to use of opioids to manage these non-pain symptoms. This situation may create a moral dilemma: is treating the
patient with opioids justified when they are not taking the opioid
for the intended purpose? Ideally, exploring symptoms more fully,
appropriately addressing underlying symptoms outside of pain,
and adequately communicating reasoning for management recommendations can help patients and families feel heard and cared for and reduce risk of patients using opioids inappropriately.
Assessment for first time opioid therapy in cancer patient: In an effort to ensure patients with cancer appropriately receive
opioids when indicated, patients with cancer-pain, or receiving
palliative care services, are excluded from the updated 2022
CDC chronic pain guidelines [17]. Patients with cancer however,
are not excluded from universal safe opioid prescribing (Table
1) [18]. While there has been documented evidence that urine
drug screens and pill counts affect cancer disease trajectories
they do help with identifying patients in need of more support
[7,19,20]. Insurance companies have broadly applied the CDC guideline principles to all patients receiving opioid therapy in varying
degrees, such as requiring prior authorizations for long-acting
opioids [21], documentation of opioid agreements, and regular urine drug screens and risk assessments. This can both support
safe opioid prescribing and act as a barrier to opioid prescribing if
clinicians do not have the resources to complete the screening or
support for completion of prior authorizations [22].
Table 1: Safe practices on opioid use, storage and disposal.
| Safe utilization |
● Use only medications
prescribed for you
● Do
not share medications
●
Follow prescriber
instructions carefully
●
Do not adjust medications
without instructions from
prescriber
● Do not
stop taking pain medications
without talking to your
medical provider
● Do
not take alcohol or other
illicit drugs when taking
pain medications. Inform
your prescriber if you do
●
Update your medication list
regularly with your
prescriber
● Do not
drive a vehicle or operate
heavy machinery when taking
pain medications
● Use
only one prescriber to
manage your pain medications
|
| Safe storage |
● Store your pain medication
in a safe place that is not
visible to other people.
Keep pain
● medications
away from young children,
adolescents, and pets
●
Place pain medications in
lockboxes
● Keep track
of your medications in a
pain/medication diary or
log
● Do not tell
others that you are taking
pain medications
●
Report lost or stolen
medications to law
enforcement personnel
|
| Safe disposal |
● Use take back programs for
unused pain medications via
local pharmacies and law
enforcement agencies
●
Dispose of unused or expired
medications using several
methods: mix with
undesirable material before
disposing in a sealed
container; flush in the
toilet.
Fold sticky ends of fentanyl
patch prior to disposal
●
Visit the DEA website
(www.deadiversion.usdoj.gov)
to look for the DEA
prescription
●
Take-Back day in your area
|
All medication prescribing whether opioids, antibiotics, antihypertensive, or other medications should be based on a detailed
clinical history, physical exam, and discussion of risks and benefits
with the patient. Opioid prescribing is under more scrutiny than
other DEA regulated medications due to the opioid crisis and risk
of harm.
Increased adoption of routine screening for distress and suicidal ideation may facilitate identification of patients at-risk for
NMOU. The incidence of mental health disorders among patients
with cancer is estimated at 35-40% [23] but these symptoms may
go undetected unless specifically assessed. As of 2015, universal
distress-screening practices have been mandated at all Commissions on Cancer-accredited cancer centers as part of standard of
care [24]. Incorporation of brief screening tools for distress, depression and anxiety such as the NCCN Distress Thermometer
[25], Patient Health Questionnaire (PHQ)-2 [26] or Generalized
Anxiety Disorder (GAD) 7 [27] may elucidate underlying mood
and anxiety symptoms contributing to NMOU, and create opportunities for conversations regarding symptom patterns and other
possible interventions.
From the vignette, Chris has more anxiety in the evening that
affects their sleep but also has periods of feeling anxious during
the daytime. They are taking their oxycodone to help with sleep
in the setting of anxiety. In this example, Chris was aware that
they were using their oxycodone for non-pain-related purposes.
In many cases, trying to distinguish between pain versus non-pain distress can be difficult due to the reciprocal nature of pain, anxiety and distress whereby the presence of anxiety and distress
can heighten the subjective experience of pain, which can then
exacerbate anxiety.
Next steps should include further assessment of Chris’s mood
and sleep symptoms, including prior management strategies, if
any, to guide further treatment options and to align with the patient’s treatment goals. Other options to offer may include antidepressant medications with anxiolytic properties, anxiolytic
medications (however should be avoided with opioids as the
combination can lead to sedation or other adverse effects). as
well as non-pharmacologic approaches such as cognitive behavioral psychotherapy, relaxation training, and meaning-centered
approaches [28].
Risk assessment tools for NMOU/SUD: Multiple assessment
tools are available to identify patients at risk of opioid misuse,
none of which have been validated in a palliative care population.
General screening tools prior to a first prescription of a controlled
substance include: the Opioid Risk Tool (ORT), Opioid Risk Tool
- Revised (ORT-R), Screener and Opioid Assessment for Patients
with Pain (SOAPP), Concurrent Opioid Misuse Measure (COMM),
Cutting down, Annoyance by criticism, Guilty feeling, Eye-openers
(CAGE) [29,30]. These are not tools to deny patients a prescription,
rather to tailor care to the needs of the patient [31]. Screening
tools can be a useful indication of those patients that may need
more support earlier in the course of treatment. Healthcare providers eliciting this history should do so with open-ended, nonjudgmental questions to encourage open conversation, and do
so universally to limit implicit bias in choosing which patients to
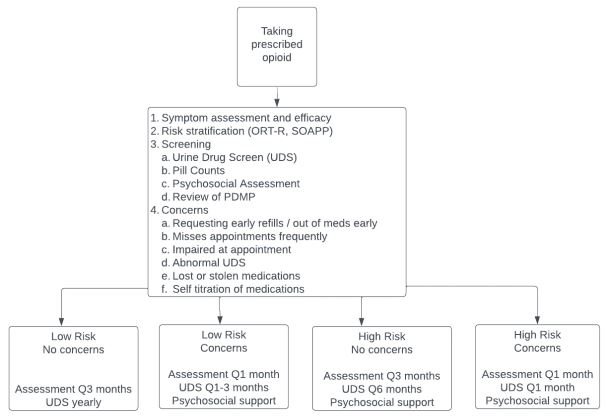
screen. Figure 1 provides an algorithm to help guide evaluation
and management of opioid therapy including suggestions for frequency of monitoring with risk scores, urine drug screens, and
when to utilize the support of the interdisciplinary team.
Case 2
Sam is a 37 year-old with stage 3a squamous cell carcinoma of
the tongue diagnosed after presenting with odynophagia. They
were prescribed oxycodone by their primary care provider 3 days
ago, 30 tablets total. As a new patient to their primary care provider, they did not disclose that they had treatment for SUD in the
past. They are calling the oncology office in pain asking for a refill.
They are asked to come in to be evaluated. Sam is asked to give a
urine sample for drug screen and results show presence of morphine, oxycodone, fentanyl and cocaine. The oncologist knows
that prescribed oxycodone does not have metabolic products that
would be detected as morphine, fentanyl, or cocaine on toxicology testing. The oncologist is worried that Sam is using non-prescribed substances suggesting that their SUD is active again, and is unsure how to proceed.
Discussion: Although the case above gives minimal information on the patient’s pain, past history, and their current situation, an oncologist may find themselves confronted with a similar
scenario. At this point, the oncologist is right to be concerned for
re-activated SUD, though this one instance of calling for an early
refill and one urine toxicology test with unexpected findings alone
cannot be interpreted as indicating an SUD diagnosis. Recognizing
a concern for SUD is critical for all clinicians who prescribe opioids
in order to comprehensively treat patients. Patients with cancer
and OUD require treatment for both conditions concurrently. Patients with OUD and cancer have a mortality that is 2.5 times the
rate of patients without an OUD [32]. Of note, patients with cancer prescribed moderate or high dose opioids are equally at risk
of overdose to patients without cancer [1]. It has only been in
the past few years that the intersection of OUD and cancer care
has gained more traction in the literature, and within the National
Comprehensive Cancer Network (NCCN) guidelines [34].
Recognizing the signs of NMOU/SUD: As oncologists who may
often initiate and maintain opioid therapy, it is crucial to identify
signs that a patient with NMOU or SUD. SUDs are characterized
by the ongoing compulsive use of substances despite harm. While
SUD are formally diagnosed by DSM5 criteria [42], they are more
easily remembered through the 4C’s mnemonic [43] (Table 5):
While a formal diagnosis of SUD using DSM-V is out of the
scope of non-addiction clinicians, best practice in the current era
includes familiarity with assessing the 4C’s to guide management
decisions for further specialty addiction assessment.
It is important to remember SUDs are chronic, treatable conditions, like diabetes or hypertension, whose natural courses may
include periods of increased disease activity, and that these periods are not signs of treatment failure or lack of motivation to be
healthier. Similarly, when patients with SUD experience increased
disease activity, such as a return to use of non-prescribed substances, or using more opioids than prescribed, clinicians should similarly view these events as indications for increasing intensity
of care, such as more frequent visits and shorter prescription fills.
It would not be compassionate or recommended to discharge a
patient from the practice, which would be punishing them.
Table 2: 4C’s for evaluating patient for possible substance use disorder.
| 4C’s |
Examples |
| Loss of Control |
Repeated requests for early
refills or being unable
to
make a prescription
last
|
| Compulsive use |
Use of substances despite
prior accidental overdose
or
use despite excess
sedation
|
| Consequences of use |
Disruption of roles or
duties with work,
parenting,
hobbies, and
relationships including use
despite concern
or
conflict with family or
clinicians over use
|
| Cravings to use |
Wanting the feeling of
relaxation from substance
when
stressed
|
Approach to the cancer patient with pain and NMOU/SUD: Oncologists commonly worry that a patient’s cancer pain will be
undertreated [22]. The question remains how to meet the patient’s needs during cancer treatment safely and effectively when
there is concurrent SUD [35]. Specialists in addiction medicine,
pain management and palliative care all have their respective
niches in management of these patients, and while some specialists find space to practice comfortably at the intersection of
cancer pain and SUD, many healthcare professionals do not feel
comfortable doing so if given other options [11]. Finding an interdisciplinary team that works together towards the goal of meeting all of the patient’s needs remains the ideal scenario, and this
may occur in the form of a symptom management “tumor board”
using an interdisciplinary team. National working groups that
give space to case discussions, access to specialists, and learning
about the intersection of pain and SUD can include programs such
as Managing Addiction and Pain in the Palliative Care Interdisciplinary Team (MAPPIT) [36]. This team has improved the comfort
level of healthcare professionals who work with patients with
SUD and serious illness. The National Clinician Consultation Center provides a national Substance Use Warmline 1 (855) 300-3595
staffed by addiction specialists for healthcare providers to call for
individual case support and resources when there is concern for
SUD.
Oncology, addiction medicine and palliative care clinicians all
value communication, team-based care, attention to quality of
life, social and structural determinants of health and ethical principles [37]. Patients with SUD deserve specialized services to optimize treatment of their SUD, management of suffering and improvement in quality of life. In health care centers that do not have
access or only limited access to these resources, oncology teams
are faced with managing symptoms or illnesses without adequate
support. A recent modified delphi study performed among specialists in addiction medicine and palliative care clinicians attempted to identify primary addiction medicine skills that would be
important to include in palliative medicine training. These skills
are also vital for oncology clinicians to obtain, especially when
they are providing primary palliative care. Please see Table 3 for
further detail [38].
Table 3: Addiction medicine skills appropriate for oncologists prescribing opioids.
|
Medical knowledge
Strategies
for preventing diversion
Understand
non-medical opioid use
Define
DSM-V criteria for OUD
|
Patient care
Manage
opioid overdose
Practice
risk mitigation
Patient-centered
decisions about opioid
prescribing
Manage
opioid withdrawal
|
Communication
Use
patient-centered and
non-judgmental language
Establish
rapport
|
Professionalism
Recognize
and address stigma
|
Systems-based practice
Coordination
of care with addiction
medicine specialists
Refer
patients for behavioral
interventions
|
When to refer to a specialist SUD/OUD: Oncologists may get
to a point in the care of their patient where they feel out of their
scope managing a patient with SUD and they should be aware
of what specialty SUD treatment is available in their area. Some
regions may have limited specialists and oncologists may assume
the primary treatment of a patient with SUD/OUD. Approaching
care for SUD/OUD as a chronic illness can help patients stabilize
and remain engaged in care for other conditions, like cancer [35].
Overwhelming evidence supports the use of medication assisted
therapy (MAT), such as methadone and buprenorphine, as medication for the treatment of opioid use disorder (MOUD) to reduce
overdose and mortality [36], and improve treatment retention
and outcomes. Buprenorphine is the only FDA approved medication for office-based treatment of OUD, and as of December 2022,
is available for all clinicians with DEA licenses to prescribe without
an X-waiver. As a partial opioid agonist, buprenorphine is also
FDA approved for the treatment of pain, which makes it a unique
opioid in the treatment of cancer-related pain for patients with
NMOU and OUD. Integrating office-based buprenorphine treatment into oncology practice ensures timely access to evidencebased care, reduces stigma associated with SUD treatment, and
allows patients to receive treatment in a familiar and accessible
setting. Resources are growing tailored to the initiation, management, and use of all formulations of buprenorphine (transdermal
patch, buccal, sublingual, and IV) in the oncology and palliative
care literature [37,38].
Table 4: PARTNERS: A structured motivational interviewing frame-
work for addressing NMOU.
Recognizing and addressing stigma of NMOU/SUD: Historical
stigma towards NMOU and SUD remains omnipresent in healthcare, resulting in punitive approaches to SUD, gaps in access to life-saving buprenorphine (less available in communities of color), and race-based disparities in pain and SUD treatment (lower
treatment of both for patients of color) [39]. Unfortunately,
health care providers continue to use stigmatizing language frequently (in the scholarly literature, in clinical notes, in education)
[40], and there is powerful intersectionality with race, gender, and
age. Specialists offering buprenorphine treatment is one way to provide anti-racist care [41,42]. Choosing to use accurate, person-first language is another way to shift practice culture. Subtle
differences in the words and phrases we use can create or dispel
stigma and have profound impacts on our patients, clinical care,
and colleagues. Table 6 shares preferred language when talking
about NMOU.
Table 5: Preferred patient-centered language for patients with NMOU/OUD.
|
Patient-centered language
|
Stigmatizing, Non-preferred
language
|
Substance use disorder
(SUD), opioid use disorder
(OUD), alcohol use
disorder
(AUD),
unhealthy/risky use
(preferred to misuse),
non-medical use,
addiction
(which can be
used to mean severe SUD per
the DSM-V
|
Substance/drug/alcohol
abuse, drug/drinking
problem. Dependence and
addiction
are both used
by patients, however
dependence clinically refers
to physiologic
withdrawal
reactions when a substance
is stopped that may occur
without a SUD
and
should not be used by
clinicians as a synonym for
SUD/addiction
|
SUD is a chronic condition
characterized by the
compulsive use of a
substance
despite
harmful consequences.
|
Addiction is a choice,
life-style, moral failing,
lack of willpower, or
personal failure
|
Person with a SUD, person
with an addiction, person
who uses drugs, person
with
injection drug use
or person who injects drugs
(PWID)
|
Addict, drug/substance
abuser, person with a drug
habit, alcoholic, IVDU (IV
drug
user), drug-seeker
|
|
Person not actively using,
person in remission from
SUD, person in recovery
|
Clean, former addict. Sober
is generally not used by
clinicians, though some
patients
use it themselves.
|
|
Substance present/not
present in urine screen
|
Dirty/clean urine. Urine
positive or negative for a
substance are also not
preferred
due to being
confusing.
|
Medications for OUD (MOUD),
Medication for addiction
treatment (MAT), Opioid
agonist
therapy (OAT)
|
Opioid replacement therapy,
opioid substitution are not
preferred as they
stigmatize
MOUD as ‘replacing one
addiction with another.’
Medication assisted
treatment
is not preferred because
MOUD alone can save lives
and no other
treatment
may be needed for some
patients.
|
Undertreated pain, risky
opioid use (e.g.
self-titration of meds,
etc.) related to
undertreated
pain (instead of
pseudoaddiction)
Using
opioids to treat non-pain
symptoms (NMOU) instead of
chemical coping
|
Pseudoaddiction, chemical
coping (these non-diagnostic
euphemisms are applied
inconsistently
and are subject to
unconscious bias in
application to patients by,
for
example, being used
by clinicians to describe
patients with OUD, for whom
the
clinician does not
feel comfortable ‘labeling’
as having OUD. This
stigmatizes OUD
and can
prevent appropriate
treatment) (23)
|
| Opioids |
Narcotics (which is used in
a legal context to refer to
multiple classes of
illegal
substances not
just opioids)
|
Recognizing patient trauma experiences and NMOU/SUD: Trauma and its effects are often invisible to the eye; however,
awareness of risk factors and inclusion of Trauma Informed Care
(TIC) can increase ability to provide person centered care with improved outcomes in the medical setting. Providers in medical settings can avoid unintentionally re-traumatizing patients with the
use of “universal trauma precautions,” which is the assumption
that every person has experienced adverse events and could be at
risk of becoming re-traumatized. Integrating universal trauma precautions into practice also encourages collaboration with patients
which leads to a higher level of and trust [43]. Adverse childhood
experience study (ACEs) provides evidence that stressful childhood events influence mental health and physical health. ACEs
such as abuse, neglect, poverty, food insecurity, violence, victimization, substance misuse in the home, incarceration of a family
member, or witnessing intimate partner violence, have all been
linked to adult morbidity and mortality. As a result, ACEs increase
the risk for these outcomes, substance use, chronic health conditions, depressive disorder, cancer, coronary heart disease, stroke,
diabetes, kidney disease, chronic obstructive pulmonary disease,
asthma and obesity [44]. Awareness of possible challenges and
integrating the principles of TIC into daily practice can reduce the
possibility of triggering and unintentionally re-traumatizing individuals [45]. The five guiding principles of TIC are safety, choice,
collaboration, trustworthiness, and empowerment. By creating a physically and emotionally safe environment, establishing trust
and boundaries, supporting autonomy and choice, creating collaborative relationships and participation opportunities, and using
a strengths and empowerment-focused perspective to promote
resilience are ways in which the principles of TIC work to reduce
re-traumatization and promote healing [46]. Team education and
collaboration using TIC are key to successfully engaging with patients and promoting quality health care experiences with vulnerable people [47].
Clinician wellness and self care in caring for patients with
NMOU/SUD: The prevalence of burnout for physicians has now
reached epidemic proportions with a prevalence as high as 50%
or greater, with oncology being higher risk [48]. Physicians have
been shown to experience three primary barriers when working
with patients with NMOU or SUD: inadequate knowledge and training, limited external community support and resources, and an
incomplete context for understanding concerning patient behaviors. 70% of physicians reported feeling negative emotions when
working with patients who had NMOU [49]. Increased contact
with patients with NMOU was significantly and positively associated with burnout scores [49,50]. The negative relationships
between bias, physician burnout, and stress induced by working
with patients with NMOU and physicians’ willingness to work with
this patient population are each exacerbated when contact with patients with NMOU is high [50]. In one study of physicians who
attended an educational conference that discussed NMOU, most
clinicians expressed concerns about under-detection and undertreatment of pain among patients with cancer. There were selfreported knowledge and confidence deficits in caring for patients
with cancer with NMOU. Seminar participation was associated
with an increase in the number of participants with self-perceived
knowledge and confidence [51]. Although intervention studies
have shown promise for the role that increased contact may have
in reducing stigma toward patients with NMOU, these interventions may not be appropriate for physicians who are experiencing
strain or who hold preexisting negative perceptions or attitudes
toward this patient population. Future interventions may need to
target bias, burnout, and stress, in addition to facilitating contact,
to increase physician willingness to work with these patients [50].
Conclusion
Patients and clinicians may both bring preconceptions about
opioid use and SUD into the patient provider relationship. When
patients are experiencing a cancer diagnosis, they may also experience NMOU or SUD. Partnering with a comprehensive interdisciplinary care team, as well as the patient and their caregiver,
may increase successful management of NMOU or SUD while undergoing cancer treatment. The tools that guide clinicians include
safe opioid prescribing guidelines, the PARTNERS communication
framework, recognizing SUD (4 C’s), and offering buprenorphine
as treatment for comorbid pain and NMOU. Recognition and understanding remains a fundamental part of caring for patients
with NMOU and SUD and preventing burnout. Communication
and consultation with palliative care, addiction medicine colleagues and following best practices increases clinician comfort and
in turn improves patient outcomes.
References
- Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022; 163(2): 328-32.
- Sabik LM, Eom KY, Sun Z, Merlin JS, Bulls HW, Moyo P, et al. Patterns and trends in receipt of opioids among patients receiving treatment for cancer in a large health system. J Natl Compr Canc Netw. 2022; 20(5): 460-467.e1.
- Swarm RA, Paice JA, Anghelescu DL, Are M, Bruce JY, Buga S, et al. Adult cancer pain, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019; 17(8): 977-1007.
- Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018; 27(5): 526-34.
- Yennurajalingam S, Edwards T, Arthur JA, Lu Z, Najera J, Nguyen K, et al. Predicting the risk for aberrant opioid use behavior in patients receiving outpatient supportive care consultation at a comprehensive cancer center. Cancer. 2018; 124(19): 3942-9.
- Jairam V, Yang DX, Yu JB, Park HS. Emergency department visits for opioid overdoses among patients with cancer. J Natl Cancer Inst. 2020; 112(9): 938-43.
- Arthur JA, Edwards T, Lu Z, Tang M, Amaram-Davila J, Reddy A, et al. Interdisciplinary intervention for the management of nonmedical opioid use among patients with cancer pain. Cancer. 2022; 128(20): 3718-26.
- Accreditation Council for Graduate Medical Education. ACGME Requirements for Graduate Medical Education in Hematology and Medical Oncology. ACGME. 2023.
- Merlin J, Young S, Arnold R, Bulls H, Childers J, Gauthier L, et al. Managing Opioids, Including Misuse and Addiction, in Patients with Serious Illness in Ambulatory Palliative Care: a qualitative study (GP765). J Pain Symptom Manage. 2020; 60(1): 284.
- Childers JW, Arnold RM. “I feel uncomfortable ‘calling a patient out’”: educational needs of palliative medicine fellows in managing opioid misuse. J Pain Symptom Manage. 2012; 43(2): 253-60.
- Janet Ho J, Jones KF, Sager Z, Neale K, Childers JW, Loggers E, et al. Barriers to buprenorphine prescribing for opioid use disorder in hospice and palliative care. J Pain Symptom Manage. 2022; 64(2): 11927.
- Sager Z, Childers J. Navigating challenging conversations about nonmedical opioid use in the context of oncology. Oncologist. 2019; 24(10): 1299-304.
- Merlin JS, Patel K, Thompson N, Kapo J, Keefe F, Liebschutz J, et al. Managing Chronic Pain in Cancer Survivors Prescribed Long-Term Opioid Therapy: A National Survey of Ambulatory Palliative Care Providers. J Pain Symptom Manage. 2019; 57(1): 20-7.
- Ferrari R. Writing narrative style literature reviews. Medical Writing. 2015; 24(4): 230-5.
- Carmichael A-N, Morgan L, Del Fabbro E. Identifying and assessing the risk of opioid abuse in patients with cancer: an integrative review. Subst Abuse Rehabil. 2016; 7: 71-9.
- Yennurajalingam S, Arthur J, Reddy S, Edwards T, Lu Z, Rozman de Moraes A, et al. Frequency of and factors associated with nonmedical opioid use behavior among patients with cancer receiving opioids for cancer pain. JAMA Oncol. 2021; 7(3): 404-11.
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022. MMWR Recomm Rep. 2022; 71(3): 1-95.
- de la Cruz M. Prescribing opioids: universal education on opioid use, storage, and disposal. Curr Anesthesiol Rep. 2020;10(4): 423-7.
- Case AA, Walter M, Pailler M, Stevens L, Hansen E. A practical approach to nonmedical opioid use in palliative care patients with cancer: using the PARTNERS framework. J Pain Symptom Manage. 2020; 60(6): 1253-9.
- Dobson M, Blackhall L. Managing Opioids in Cancer Patients at High Risk for Substance Use Disorders: Experience from an Outpatient Palliative Care Clinic (RP311). J Pain Symptom Manage. 2022; 63(6): 1073.
- Keast SL, Kim H, Deyo RA, Middleton L, McConnell KJ, Zhang K, et al. Effects of a prior authorization policy for extended-release/long-acting opioids on utilization and outcomes in a state Medicaid program. Addiction. 2018.
- Schenker Y, Hamm M, Bulls HW, Merlin JS, Wasilko R, Dawdani A, et al. This Is a Different Patient Population: Opioid Prescribing Challenges for Patients With Cancer-Related Pain. JCO Oncol Pract. 2021; 17(7): 1030-7.
- Caruso R, Breitbart W. Mental health care in oncology. Contemporary perspective on the psychosocial burden of cancer and evidence-based interventions. Epidemiol Psychiatr Sci. 2020; 29: 86.
- American College of Surgeons. Cancer Program Standards: Ensuring Patient-Centered Care. 2016th ed. Chicago, IL: American College of Surgeons. 2016.
- Riba MB, Donovan KA, Ahmed K, Andersen B, Braun Ii, Breitbart WS, et al. NCCN guidelines® insights: distress management, version 2.2023. J Natl Compr Canc Netw. 2023; 21(5): 450-7.
- Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007; 22(11): 1596-602.
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006; 166(10): 1092-7.
- Shoval G, Balicer RD, Feldman B, Hoshen M, Eger G, Weizman A, et al. Adherence to antidepressant medications is associated with reduced premature mortality in patients with cancer: A nationwide cohort study. Depress Anxiety. 2019; 36(10): 921-9.
- Lau J, Mazzotta P, Fazelzad R, Ryan S, Tedesco A, Smith AJ, et al. Assessment tools for problematic opioid use in palliative care: A scoping review. Palliat Med. 2021; 35(7): 1295-322.
- Cheatle MD, Compton PA, Dhingra L, Wasser TE, O’Brien CP. Development of the Revised Opioid Risk Tool to Predict Opioid Use Disorder in Patients with Chronic Nonmalignant Pain. J Pain. 2019; 20(7): 842-51.
- Jones KF, Malinowski J, Paice J, Childers J, Bulls HW, Morrison J, et al. Opioid prescribing considerations in patients with cancer and substance misuse and use disorder: a scoping review protocol. JBI Evid Synth. 2022.
- Kale S, Russell D, Edwards B, Curenton F, Parker G, John ASt, et al. A Journey Worth Taking Together: Our Experience Starting an Ambulatory Palliative Clinic to Care for Patients with Cancer and Substance Misuse/Use Disorders (TH109A). J Pain Symptom Manage. 2023; 65(3): 252.
- Bhatia A, Kara J, Janmohamed T, Prabhu A, Lebovic G, Katz J, et al. User Engagement and Clinical Impact of the Manage My Pain App in Patients With Chronic Pain: A Real-World, Multi-site Trial. JMIR Mhealth Uhealth. 2021; 9(3): 26528.
- Col N, Hull S, Springmann V, Ngo L, Merritt E, Gold S, et al. Improving patient-provider communication about chronic pain: development and feasibility testing of a shared decision-making tool. BMC Med Inform Decis Mak. 2020; 20(1): 267.
- Abuse S. Medications for Opioid Use Disorder (No. PEP20-02-01–006; Treatment Improvement Protocol (TIP) Series 63). 2021.
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020; 3(2): 1920622.
- Jones KF, Merlin JS. Approaches to opioid prescribing in cancer survivors: Lessons learned from the general literature. Cancer. 2022; 128(3): 449-55.
- Neale KJ, Weimer MB, Davis MP, Jones KF, Kullgren JG, Kale SS, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2022.
- Wakeman SE, Rich JD. Barriers to medications for addiction treatment: how stigma kills. Subst Use Misuse. 2018; 53(2): 330-3.
- Sedney CL, Dekeseredy P, Singh SA, Holbein M. Stigmatizing language expressed towards individuals with current or previous OUD who have pain and cancer: A qualitative study. J Pain Symptom Manage. 2023.
- Lagisetty PA, Ross R, Bohnert A, Clay M, Maust DT. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 2019; 76(9): 979-81.
- Nguyen T, Ziedan E, Simon K, Miles J, Crystal S, Samples H, et al. Racial and Ethnic Disparities in Buprenorphine and Extended-Release Naltrexone Filled Prescriptions During the COVID-19 Pandemic. JAMA Netw Open. 2022; 5(6): 2214765.
- Schachter CL, Radomsky NA, Stalker CA, Teram E. Women survivors of child sexual abuse. How can health professionals promote healing? Can Fam Physician. 2004; 50: 405-12.
- Merrick MT, Ford DC, Ports KA, Guinn AS, Chen J, Klevens J, et al. Vital Signs: Estimated Proportion of Adult Health Problems Attributable to Adverse Childhood Experiences and Implications for Prevention - 25 States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2019; 68(44): 999-1005.
- Jones Q, Johnston B, Biola H, Gomez S, Crowder C. Implementing standardized substance use disorder screening in primary care. JAAPA. 2018; 31(10): 42-5.
- University of Buffalo. The Institute on Trauma and Trauma-Informed Care (ITTIC) [Internet]. University of Buffalo - Buffalo Center for Social Research. 2023. https://socialwork.buffalo.edu/socialresearch/institutes-centers/institute-on-trauma-and-trauma-informed-care.html.
- Owens L, Terrell S, Low LK, Loder C, Rhizal D, Scheiman L, et al. Universal precautions: the case for consistently trauma-informed reproductive healthcare. Am J Obstet Gynecol. 2022; 226(5): 671-7.
- Rothenberger DA. Physician Burnout and Well-Being: A Systematic Review and Framework for Action. Dis Colon Rectum. 2017; 60(6): 567-76.
- Dhanani LY, Harris EL, Mirto J, Franz B. Barriers to Working with Patients Who Misuse Opioids and Physician Burnout: Implications for Medical Education. Subst Use Misuse. 2022; 57(8): 1177-84.
- Dhanani LY, Franz B, Hall TK. Revisiting the relationship between contact and physician attitudes toward patients with opioid use disorder. Addict Behav Rep. 2021; 14: 100372.
- Arthur J, Edwards T, Lu Z, Hui D, Fellman B, Bruera E. Health care provider attitudes, beliefs, and perceived confidence in managing patients with cancer pain and nonmedical opioid use. J Pain Symptom Manage. 2021; 61(1): 128-135.e6