Introduction
Hepatocellular carcinoma (HCC) is a common malignant tumors with a very poor prognosis [1]. Surgery remains the preferred treatment for HCC [2]. However, many patients are already
advanced once diagnosed and lose the opportunity of radical
resection [3]. Sorafenib is multi-kinase inhibitor commonly used
in advanced HCC [4]; In the past few decades, is the first choice
for the fight against advanced HCC. However, the patient’s median overall survival was short. Therefore, a search for novel
therapeutic strategies is necessary. Immunotherapy has brought about a paradigm shift in cancer treatment in recent years [5].
The liver offers a unique opportunity to utilize modifications in
immunomodulatory pathways as a potential therapeutic strategy
for HCC. Hepatic arterial infusion chemotherapy (HAIC) is another
palliative care form for advanced HCC [6]. It is indicated for nonresectable, refractory to TACE, or tumors with portal vein tumor
thrombus (PVTT).
More and more clinical research failures have hit us hard, until a clinical study named IMbrave150, published in the New England Journal of Medicine in 2020 [7]. It has opened up a new era of combination therapy, breaking the pattern of only a single
mode of advanced liver cancer for more than ten years, making
us realize that for the treatment of patients with advanced HCC,
the effect of single treatment is very limited. The combination
therapy is the future [8-10]. However, the prognosis of advanced
HCC with tumor thrombosis (VP4) is still unsatisfactory. This case
report presents a patient who obtained a good response to antiPD-1 therapy combined with HAIC for VP4.
Case presentation
A 56-year-old woman with advanced HCC with tumor thrombosis has been treated with oral Entecavir antiviral therapy and
sorafenib since December 15, 2021. However, after oral sorafenib
half a month later, the patient experienced hematemesis and systemic rash reactions and was referred to our hospital. The patient
was alert and clear, with a height of 153 cm and a weight of 52 kg.
Physical examination showed rash on the skin of the whole body.
The abdomen was soft and the liver and spleen were not touched.
HBs antigen and HBV-DNA is positivity. Child–Pugh status was A
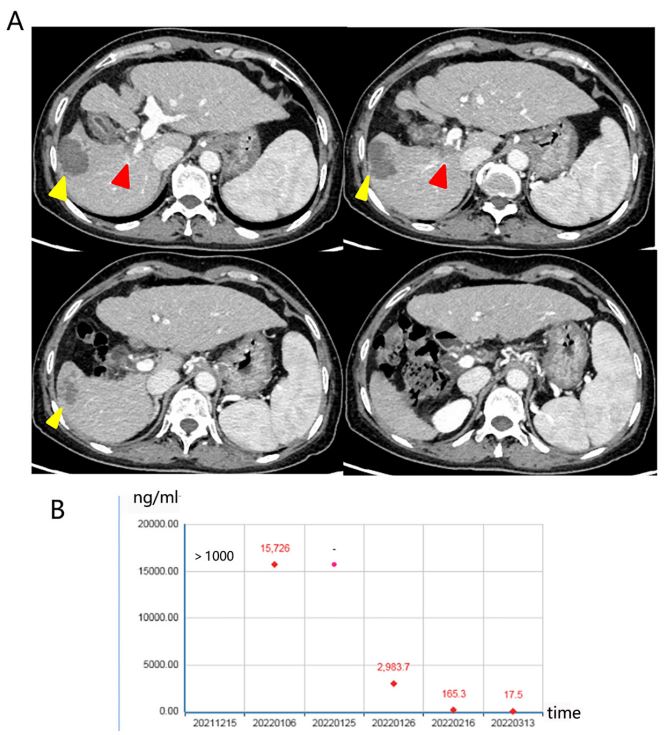
(Table 1). The serum levels of tumor marker were elevated (AFP
15,726 ng/mL), and a mass lump of 8.6 cm in the right lobe of the
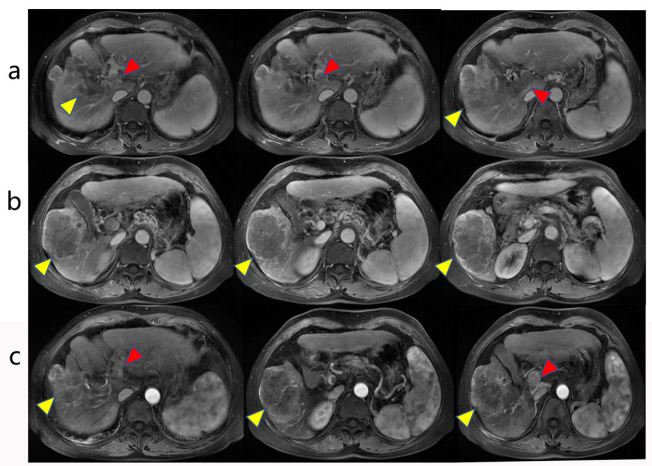
liver with PVTT in the main branch (Figure 1).
After the MDT, it was decided to discontinue sorafenib and
use anti-PD-1 therapy combined with HAIC for her. The patient
received HAIC therapy with FOLFOX4 scheme (oxaliplatin 150 mg
infusion for 2h, leucovorin 200 mg/m2
infusion for 2h, and fluorouracil 400 mg/m2
in bolus within 10 min, and then 2,400 mg/
m2
continuous infusion for 46h) on January 1, January 27, and
February 18, 2022, respectively. The patient was treated with
anti-PD-1 therapy (Tislelizumab 200 mg infusion) on January 9,
January 29, and February 20, 2022, respectively. About 2 months
of therapy, the tumor size was reduced from 8.6 cm to 4 cm of
the patient, and PVTT decreased from Vp4 to Vp3 (Figures 2A).
The serum levels of tumor marker were decreased during treatment (Figure 2B). She was assessed for surgical removal, and right
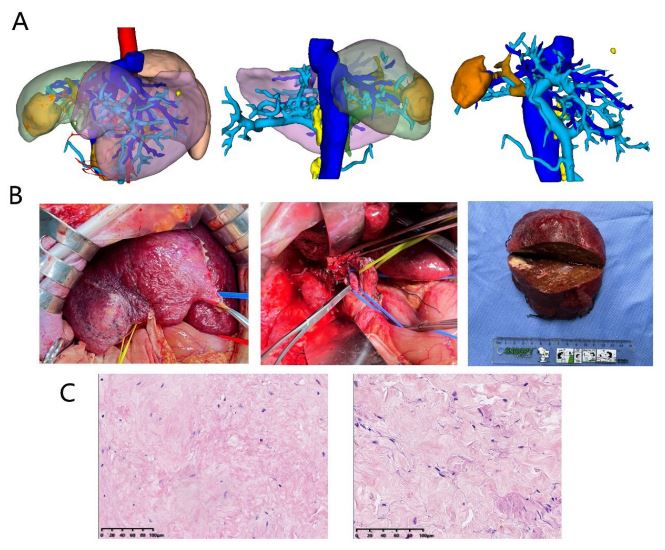
hepatectomy plus portal vein reconstruction was performed on
March 19, 2022 (Figures 3A,B). On pathological examination, tumor cells in the entire liver tumor and portal vein cancer thrombus are completely necrotic (Figures 3C). The patient recovered
well after surgery and was discharged from the hospital for one
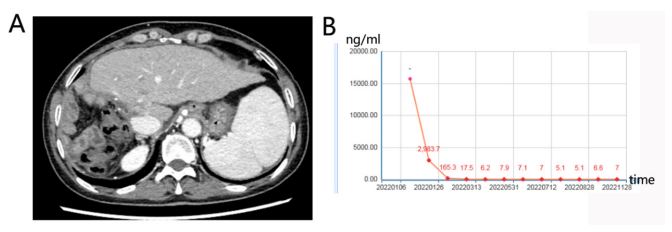
week. A complete response was achieved and the patient remained disease-free as of the last follow-up on November 28, 2022.
The AFP remained within normal range and CT showed no tumor
remnants (Figures 4).
Table 1: Laboratory examinations.
| CBC |
|
| WBC |
3.08×109 /L |
| RBC |
3.85×1012 /L |
| Hb |
111 g/L |
| PIt |
62× 109 /L |
| PT |
80% |
| PT-INR |
1.12 |
| TBil |
20.84 μmol/L |
| AST |
35.64 U/L |
| ALT |
28.77 U/L |
| ALP |
62 U/L |
| Na |
135.53 mmol/L |
| K |
4.2 mmol/L |
| Cl |
97 mmol/L |
| Alb |
38.5 g/L |
| AFP |
15,726 ng/mL |
| HBs antigen |
(+) |
| HBs antibody |
(−) |
| HBe antibody |
(+) |
| HBV-DNA |
4.66×103 IU/mL |
| HCV antibody |
(−) |
| Child-pugh score |
5 |
| Child-pugh grade |
A |
AFP: A-fetoprotein; Alb: Albumin; ALP: Alkaline phosphatase;
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; Cr:
Creatinine; Hb: Hemoglobin; PT: Prothrombin Time; Plt: Platelets; TBil:
Total Bilirubin; PT-INR: international normalized ratio of prothrombin
time; RBC: Red Blood Cells; CBC: Complete Blood Cell Count; WBC: White
Blood Cells; HCV: Antibody hepatitis C Virus antibody; HBs: Antigen
hepatitis B surface antigen.
Discussion
Recently, significant progress has been made in treatment of
HCC [11]. Pharmacotherapy, particularly immunotherapy combined with anti-angiogenic drugs for intermediate and advanced
HCC, yields an objective response rate of about 30 percent, and
median survival is improved to about 20 months [12,13]. Clinical
studies on translational therapy after systemic therapy have also
been reported. In New England Journal of Medicine, the complete
study data of the Phase III clinical trial IMbrave150 study was officially published, and the anti-angiogenic drug bevacizumab combined with the PD-L1 inhibitor Atezolizumab, achieved success for
treatment with advanced HCC [7]. It is recognized that the effect
of single treatments is limited and combination therapy is a promising direction for breakthroughs in advanced HCC.
Significant progress has been made by some researchers in the
field of HAIC for advanced liver cancer. A multicenter randomized
controlled trial (RCT) study showed that the overall response rate
(ORR) of patients with liver cancer treated with PVTT was significantly higher than that of sorafenib (27.6% vs. 3.4%, P=0.001)
[14]. Tyrosine Kinase Inhibitor (TKI) combined with PD-1 inhibitor,
the conversion resection rate was 42.4%, Zhu et al [15] reported
that 63 patients with unresectable HCC were treated with TKI
combined with PD-1 inhibitors, and the conversion resection rate
was 15.9%. Sorafenib is a multi-kinase inhibitor used to treat patients with HCC. In this case, the patient developed hematemesis
with a systemic rash half a month after oral sorafenib. The side effects led the patient to discontinuation sorafenib. As a result, it
was decided to use anti-PD-1 therapy combined with HAIC for this
patient. After about 2 months of the therapy, the CT scan showed
a significant reduction in the tumor size, and PVTT decreased from
Vp4 to Vp3. The patient eventually achieved radical resection and
on pathological examination, tumor cells in the entire liver tumor
and portal vein cancer thrombus are completely necrotic.
Conclusion
In summary, the use of multidisciplinary, multi-method combination treatment mode has become an inevitable trend, the
treatment process should pay more attention to the patient’s individualized treatment, in the affirmation of the efficacy of surgery
for advanced patients, but also highlight the role of comprehensive treatment, according to the different conditions of patients to
provide the best treatment plan, for more patients with advanced
HCC to bring hope.
Declarations
Funding: This study was supported Jiangxi Province Health
Commission Science and Technology Pla (Award Number
202210656).
Compliance with ethical standards: This study was approved
by the medical ethics committee of the Second Affiliated Hospital of Nanchang University and obtained the written informed
consent of patients to publish clinical details and images.
Competing interests: The patient signed the informed consent
form approved by the institutional review committee and agreed
to the publication of the journal.
The authors report no conflicts of interest in this work.
References
- Pan W LiW, Zhao J, Huang Z, Zhao J, Chen S, et al. Lncrna-Pdpk2p Promotes Hepatocellular Carcinoma Progression through the Pdk1/Akt/Caspase 3 Pathway. Molecular oncology. 2019; 13(10): 2246-58. Epub 2019/08/02. doi: 10.1002/1878-0261.12553.
- Guarino M, Caporaso N, Morisco F. Liver Resection Is Always a Good Choice for Hepatocellular Carcinoma (Hcc) Patients Regardless of Barcelona Clinic Liver Cancer (Bclc) Stage: The Therapeutic Hierarchy. Ann Transl Med. 2020; 8(20): 1282. Epub 2020/11/20. doi: 10.21037/atm-20-6004.
- Yang JD, Heimbach JK. New Advances in the Diagnosis and Management of Hepatocellular Carcinoma. BMJ. 2020; 371: 3544. Epub 2020/10/28. doi: 10.1136/bmj.m3544.
- Kosaka Y, Kawaoka T, Aikata H, Suehiro Y, Yamaoka K, Ando Y, et al. A Case of Advanced Hcc Treated with Lenvatinib after Hepatic Arterial Infusion Chemotherapy Combined with Radiation Therapy Treatment for Portal Vein Tumor Thrombosis in the Main Trunk. Clin J Gastroenterol. 2020; 13(5): 839-43. Epub 2020/01/25. doi: 10.1007/s12328-020-01093-9.
- Mamdani H, Wu H, O’Neil BH, Sehdev A. Excellent Response to Anti-Pd-1 Therapy in a Patient with Hepatocellular Carcinoma: Case Report and Review of Literature. Discov Med. 2017; 23(128): 331-6. Epub 2017/07/18.
- Ishizaki M, Kaibori M, Matsui K, Nakatake R, Matsushima H, Sakaguchi T, et al. [a Case of Curative Resection for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombus after Hepatic Arterial Infusion Chemotherapy]. Gan To Kagaku Ryoho. 2012; 39(12): 1991-3. Epub 2012/12/27.
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020; 382(20):1894-905. Epub 2020/05/14. doi: 10.1056/NEJMoa1915745.
- Hu X, Li R, Li Q, Zang M, Yuan G, Chen J. Interaction between Baseline Hbv Loads and the Prognosis of Patients with Hcc Receiving Anti-Pd-1 in Combination with Antiangiogenic Therapy Undergoing Concurrent Taf Prophylaxis. BMC Infect Dis. 2022; 22(1): 614. Epub 2022/07/15. doi: 10.1186/s12879-022-07602-0.
- Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X, et al. Combination Therapy of Sorafenib and Tace for Unresectable Hcc: A Systematic Review and Meta-Analysis. PloS one. 2014; 9(3): 91124. Epub 2014/03/22. doi: 10.1371/journal.pone.0091124.
- Dong Y, Wong JSL, Sugimura R, Lam KO, Li B, Kwok GGW, et al. Recent Advances and Future Prospects in Immune Checkpoint (Ici)-Based Combination Therapy for Advanced Hcc. Cancers (Basel). 2021; 13(8). Epub 2021/05/01. doi: 10.3390/cancers13081949.
- Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, et al. Chinese Expert Consensus on Conversion Therapy for Hepatocellular Carcinoma (2021 Edition). Hepatobiliary Surg Nutr. 2022; 11(2): 227-52. Epub 2022/04/26. doi: 10.21037/hbsn-21-328.
- Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (Rescue): A Nonrandomized, Open-Label, Phase Ii Trial. Clin Cancer Res. 2021; 27(4): 1003-11. Epub 2020/10/23. doi: 10.1158/1078-0432.CCR-20-2571.
- Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020; 38(26): 2960-70. Epub 2020/07/28. doi: 10.1200/JCO.20.00808.
- Choi JH, Chung WJ, Bae SH, Song DS, Song MJ, Kim YS, et al. Randomized, Prospective, Comparative Study on the Effects and Safety of Sorafenib Vs. Hepatic Arterial Infusion Chemotherapy in Patients with Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancer Chemother Pharmacol. 2018; 82(3): 469-78. Epub 2018/07/10. doi: 10.1007/s00280-018-3638-0.
- Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-Pd-1 Antibody Combinations. Liver Cancer. 2021; 10(4): 320-9. Epub 2021/08/21. doi: 10.1159/000514313.