Introduction
One of the deadliest women’s cancers is ovarian cancer. Annually, there were 295.414 new cases with 184.799 deaths in
2018 worldwide. The incidence in Indonesia of 9.7 per 100.000
[1]. Furthermore, it is hard to detect and prevent because mostly
(70%) of patients present with an advanced stage [2]. Standard
therapy with cytoreductive surgery followed by platinum-based
chemotherapy has a high recurrence rate. It has a 77.4% chemo
sensitivity rate and an 18.1% chemo-resistance rate [3] with the
Progression-Free Survival (PFS) was 12 months and Overall Survival (OS) was about 30 months [4,5]. Cancer Stem Cells (CSCs)
have a role in the low survival rate [6].
The chemo-resistance resulted from Cancer Stem Cells (CSCs).
CSCs have an essential role in the initiation, tumor growth, metastasis, and recurrence which leads to chemotherapy resistance [7].
Studies about the DNA Damage Response (DDR) in the tumorigenic process found that DDR was correlated with the formation
of CSCs and chemo-resistant cells. The DDR pathway consists of
Post Replication Repair (PRR), Nucleotide Excision Repair (NER),
Fanconi anemia, etc. The PRR involves several proteins such as E2
Ubiquitin-Conjugating Enzymes (UBE2) protein RAD6 [8].
RAD6 is an UBE2 protein required for DNA regulation, repair,
proliferation, and mutagenesis. Cell transformation and mitotic
abnormalities associated with RAD6 expression. RAD6 overexpression leads to elevated Cancer Stem Cell (CSCs) markers and
signaling pathways components that enhance stemness function, chemo-resistance, metastasis, and cancer progression [9].
The RAD6 is associated with chemo-resistance and poor clinical
prognosis in ovarian cancer. Somasagara et al. reported that RAD6
expression <5 and >5 was associated with 37.5% and 70% recurrence, respectively [9]. We want to see the expression of RAD6
in ovarian cancer patients’ tissue and blood after chemotherapy
which has never been conducted before. Our objective is to find
relationships between RAD6 with chemotherapy response in
ovarian cancer and its ability to predict ovarian cancer chemotherapy response.
Materials and methods
Study design
This study design is an ambispective cohort (prospective and
retrospective cohort) at the obstetrics-gynecology and anatomical pathology department of Cipto Mangunkusumo Hospital,
Tarakan Hospital, Dharmais Hospital, and Fatmawati Hospital for
two years from February 2018 until February 2022.
Participants
The research subjects were patients with ovarian carcinoma
inclusion, stage II-IV ovarian epithelial cancer patients, and were
willing to participate in the study. The sample exclusion criteria
were pregnant patients and patients diagnosed with other types
of cancer. The number of samples in this study was 32 people in
each group with consecutive sampling methods to minimize selection bias.
Data collection
Ovarian cancer patients will undergo cytoreductive debulking
and histopathological examination. If the histopathology result is malignant, chemotherapy will be given for six series followed by
six months of observation. After the observation, we determined
therapy response with the RECIST Criteria (Response Criteria in
Solid Tumors) and then classify it into chemo-resistant or chemo
sensitive groups. The patient will perform Flow cytometry blood
tests to examine the expression of RAD6 (prospective study),
while an immunohistochemistry examination will be performed
on ovarian cancer tissue (retrospective study). We also collected
demographic data, cancer stage, surgery type, chemotherapy response, tumor cell differentiation (cancer stage), cancer histopathology, cancer size, cancer residue, ascites, lymph node metastasis, and serum Ca-125 levels. FIGO criteria were being used for
cancer staging.
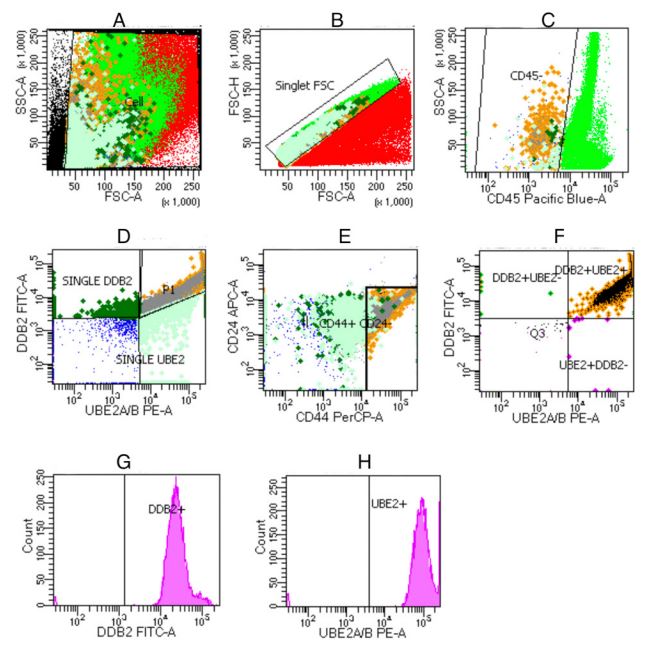
Flow cytometry method
Blood was taken from peripheral blood veins at five ml and
centrifugated with 50 µL was left. Their markers identified the expression CD44+
/CD24-
. Samples were reacted with fluorescent-labeled antibody against RAD6 (monoclonal anti-human) labeled as
PE. The reagents were removed for leukocytes with CD45 labeled
pacific blue. The samples in the Falcon tube were added with 2,5
µL of RAD6 marker, then incubated for 15 minutes in the dark at
room temperature. After incubation, cells were lysed using 300
µL of lysing solution, then set again for 15 minutes in a dark room
and at room temperature. Next, 1 mL of facs flow solution was
added and centrifuged at 500 g for 5 minutes, then added with
500 µL perm wash buffer and centrifuged at 500 g for 5 minutes.
To be more optimal, 1 mL perm wash buffer was added again and
centrifuged at 500 g for 5 minutes. The last step was to add 200
µL of 1% paraformaldehyde in Phosphate-Buffered Saline (PBS).
After that, the analysis was carried out using a flow cytometer
using four fluorochrome colors.
Flow cytometry cell count
Cell identification was carried out using an automated flow
cytometer (BD Facs Calibur). CSCs were identified through the
positive expression of RAD6markers. Protein percentage is the
percentage of expression of protein markers RAD6 in the blood.
Immunohistochemistry slide preparations
The examination used paraffin block specimens. In each case,
eight preparations were made from paraffin blocks which were
cut with a microtome with a thickness of 3 cm and placed on a
poly-L-lysine-coated slide, then dried at 37°C and heated on a
slide warmer at 600°C for 30 minutes. Then, it deparaffinized
using graded xylol (xylol I, II, and III, for 5 minutes each) and rehydrated with serial alcohol (96% and 80% alcohol, respectively, for
4 minutes), then washed with running water for 5 minutes. Furthermore, we carried a blocking method to inhibit endogenous
peroxidase activity using 1.5% hydrogen peroxide in methanol for
10 minutes at room temperature. It was rewashed with running
water for 5 minutes. The next step was pretreatment using Tris
EDTA acid (pH 9.0) in a decloaking chamber at 960 degrees Celsius
for 10 minutes, cooled for 45 minutes, and washed in PhosphateBuffered Saline (PBS) at pH 7.4. After that, we carried a blocking
method to non-specific protein using background sniper universal
for 15 minutes.
Detection of RAD6 markes used specific antibodies against
RAD6 (Monoclonal anti-RAD6). The preparations were incubated with a primary RAD6 antibody (1:500 dilution). After one hour, it
was washed with PBS (pH 7.4) for 5 minutes. Each preparation
was then incubated with a secondary antibody against biotinlabeled mouse immunoglobulin (Trekkie Universal Link) for 20
minutes and then washed again in PBS (pH 7.4) for 5 minutes.
Next was incubation with trackAvidin-HRP labeled for 15 minutes,
then washed in PBS (pH 7.4) for 5 minutes. Then, Diaminobenzene (DAB) was mixed with 1 mL of a substrate and vortexed for
15 seconds. The substrate containing DAB was dripped onto the
preparation, incubated for 2 minutes, and washed with running
water for 10 minutes.
Next, it was counterstained with CAT (Counterstain Kit) hematoxylin for 5 seconds and washed with running water for 5 seconds. The preparation was immersed in saturated lithium carbonate (5% in distilled water) for 5 seconds, then washed with
running water for 5 minutes. The dehydration process was carried
out with graded alcohol (80%, 96%, absolute, absolute) for 5 minutes each and clearing with graded xylol (xylol I, II, and III) for 5
minutes each. The preparation was closed using a mounting solution and a cover glass. Each smear included an internal positive
control on the stromal tissue and a negative control without primary antibodies. Positive and negative controls were performed
on the same tissue as the tumor tissue.
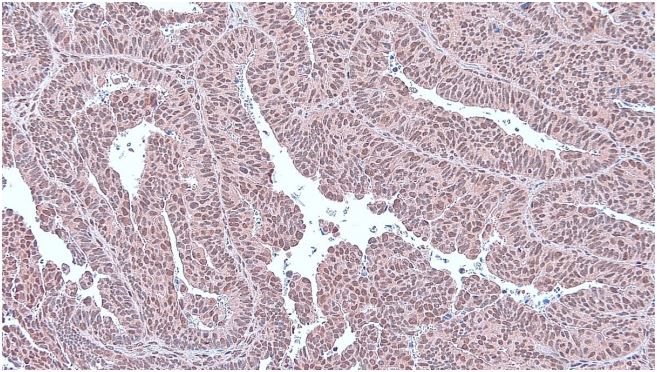
Immunohistochemistry
The immunohistochemistry preparations were observed using
a Leica ICC 50 HD microscope. Positive RAD6 was seen in the staining of the cytoplasm and the nucleus of tumor cells. Immunohistochemistry assessment classified as 0: Negative expression, 1:
Weak expression, 2: Moderate expression, and 3: Strong expression. Next, it is classified into low expression and high expression.
The low expression has 0-1 while the high expression has a 2-3
value [10].
Statistical analysis
We conduct univariate, bivariate, and multivariate analyses.
Each categorical variable was tested with the chi-square or alternative Fisher test. ROC and AUC curves were used to test the
flow cytometry and immunohistochemistry RAD6 variable as a
predictor of therapy response to ovarian cancer. We performed a
multivariate analysis to compare the power between RAD6 flow
cytometry and immunohistochemistry to predict ovarian cancer
chemo-resistance. Missing data and lost follow-up patients will be
discarded from the sample.
Ethical clearance:Research ethics approval was obtained from
the Health Research Ethics Committee of the Universitas Indonesia, Cipto Mangunkusumo Hospital.
Result
Basic participants characteristics
We have 32 samples in each group. All samples had undergone
chemotherapy with 32 (50%) chemo-resistance patients and 32
(50%) chemo sensitive patients for each flow cytometry and immunohistochemistry study. There is no missing data or lost follow-up patients after 6 months of observation. The distribution of
profiles and clinical characteristics of ovarian cancer patients can
be seen in Table 1.
Flow cytometry of ovarian cancer
The flow cytometry results example is presented in Figures 2
and 3. The proportion of RAD6 values was calculated based on the
percentage of the total cells. RAD6 was highly expressed in chemo-resistance ovarian cancer patients like in the previous studies.
Bivariate analysis
RAD6 Flow cytometry RAD6 has OR 4.76 and 2.45, respectively
while immunohistochemistry RAD6 has OR 52.2 and 6.12. Thus,
RAD6 immunohistochemistry has a higher OR and RR value. The
complete data is shown in Table 2. The data quietly found that
chemo-resistance ovarian cancer patients have high RAD6 expression.
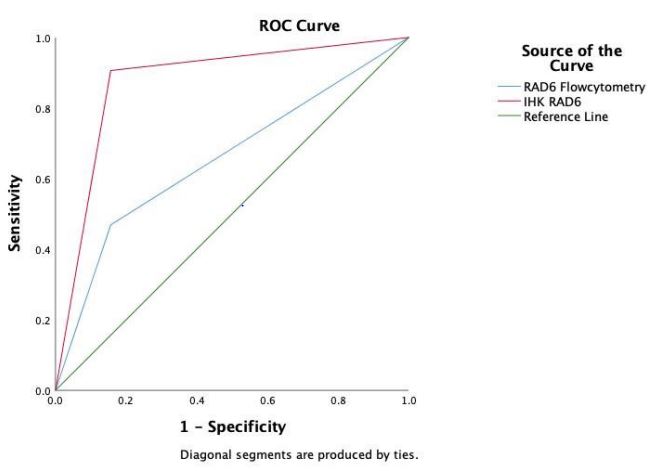
ROC and AUC curves
ROC curve data were presented in Figure 1 while the AUC analysis was presented in Table 3. The AUC value of the RAD6 flow
cytometry is 0.656, which means it has a poor level of accuracy,
but the value is significant (p<0,05). The sensitivity is 46 %, and
its specificity is 84% for detecting chemo-resistance. RAD6 immunohistochemistry had a better AUC of 0.875 (good accuracy),
significant (p<0,05), with a better sensitivity of 90% and better
specificity of 84%. The data showed that the RAD6 immunohistochemistry has better ROC curve and AUC value.
Multivariate analysis
We conducted logistic regression and the results are shown
in Table 4. We found from this calculation that immunohistochemistry data of RAD6 has a better result compared with flow cytometry data of RAD6. It means that RAD6 immunohistochemistry
is a better predictor of ovarian cancer chemo-resistance in this
research.
Table 1: Essential clinical characteristics of ovarian cancer patient.
| Variable |
Number (%) |
• Chemoresistant
• Chemosensitive |
32(50)
32(50) |
Age (years old)
• <40
• 40-50
• >50 |
4(6,3)
19(29,7)
41(64,1) |
Ca-125
• ≤35
• >35 |
30(46,9)
34(53,1) |
Ovarian cancer stage
• Early stage: II
• Advance stage: III - IV |
5(7,8)
59(92,2) |
Surgery type:
• Optimal Debulking
• Suboptimal Debulking |
56(87,5)
8(12,5) |
Differentiation/cancer grade
• Good
• Intermediate
• Poor |
13(20,3)
16(25,0)
35(53,1) |
Tumor histology type
• Serous
• High-grade serous
• Mucinous
• Endometrioid
• Clear cell
• Others |
24(37,5)
14(21,9)
3(4,7)
12(18,8)
10(15,6)
1(1,6) |
Lymph nodes metastasis
• Positive
• Negative |
32(50)
32(50) |
Ascites
• Positive
• Negative |
36(56,3)
28(43,7) |
Tumor size
• 5 cm
• 5-10 cm
• >10 cm |
17(26,6)
15(23,4)
32(50) |
Tumor residue
• < 1cm
• > 1cm |
56(87,5)
8(12,5) |
Table 2: Bivariate analysis of the variables in ovarian cancer patients.
| Variable |
Therapy response |
P value |
OR
(CI 95%) |
RR
(CI 95%) |
| Chemo resistant (%) |
Chemo sensitive (%) |
RAD6 flow cytometry
• High (≥32.692)
• Low (<32.692) |
15(46.9)
17(53.1) |
5(15.6)
27(84.4) |
0.007* |
4.76
(1.46-15.5) |
2.45
(1.11-5.43 |
RAD6 immunohistochemistry
• High (≥10%)
• Low (<10%) |
29(90.6)
3(9.4) |
5(15.6)
27(84.4) |
0.000* |
52.20
(11.3-239) |
6.12
(2.7-13.8) |
Ca-125 Level
• ≤35
• >35 |
2(6,25)
30(93,75) |
28(87.5)
4(12,5) |
0,001* |
105
(17-618) |
7,93
(3.14-20.0) |
Ovarian cancer stage
• Early stage: II
• Advance stage: III - IV |
1(3,13)
31(96,87) |
4(12,5)
28(87,5) |
0,162 |
4.42
(0.47-42) |
1.68
(1.7-4.4) |
Surgery type
• Optimal Debulking
• Suboptimal Debulking |
25(84,4)
7(15,6) |
31(96,87)
1(3,13) |
0,023* |
8.68
(1.0-75.3) |
4.43
(0.69-28.12) |
Differentiation/cancer grade
• Good
• Intermediate - Poor |
6(18,75)
26(81,25) |
7(21,88)
25(78,12) |
0,760 |
1,21
(0.36-4.11) |
1.09
(0.62-1.96) |
Lymph nodes metastasis
• Positive
• Negative |
21(65,63)
11(34,37) |
11(34,37)
21(65,63) |
0,012* |
3.65
(1.29-10.2) |
1.91
(1.1-3.2) |
Ascites
• Positive
• Negative |
18(56,25)
14(43,75) |
14(43,75)
18 (56,25) |
1,000 |
1
(0.37-2.68) |
1
(0.61-1.64) |
Tumor size
• ≤5 cm
• >5 cm |
6(18.8)
26(81.2) |
8(25)
24(75) |
0.545 |
1.44
(0.44-4.7) |
1.19
(0.69-2.04) |
Tumor residue
• <1cm
• > 1cm |
25(84,4)
7(15,6) |
31(96,87)
1(3,13) |
0,023* |
8.68
(1.0-75.3) |
4.43
(0.69-28.12) |
Note: *: p<0,05, Significant results.
Table 3: AUC analysis of RAD6 flow cytometry and immunohistochemistry.
| Variable |
AUC |
SD |
95% CI |
Sensitivity (%) |
Specificity (%) |
P value |
| RAD6 flow cytometry |
0.656 |
0.069 |
0.521-0.792 |
46 |
84 |
0.032* |
| RAD6 immunohistochemistry |
0.875 |
0.048 |
0.781-0.969 |
90 |
84 |
0.000* |
Note: *: p<0,05, Significant.
Table 4: Logistic regression of RAD6 flow cytometry and immunohistochemistry.
| No |
Variables |
Beta value (β) |
Standard deviation |
Wald |
p value |
Exp (B) |
95% CI |
| 1 |
RAD6 flow cytometry |
2.662 |
1.174 |
5.143 |
0.023* |
14.323 |
1.435-142.9 |
| 2 |
RAD6 immunohistochemistry |
4.635 |
1.106 |
17.570 |
0.000* |
103.077 |
11.79-900.5 |
| Constant |
|
-8.038 (β0) |
2.086 |
14.843 |
0,000 |
|
- |
Note: *: p<0,05, Significant.
Discussion
RAD6 is a Ubiquitin-Conjugating Enzyme E2 (UBE2), an enzyme
that play a role in the occurrence of chemo-resistance in ovarian
cancer. RAD6 plays a role in DNA repair and regulates gene expression through modification of histone pro-transcriptions [11].
Humans have two RAD6 proteins (RAD6A & B or UBE2A & UBE2B),
which are often overexpressed in various tumor types [9].
The mechanism of RAD6 in increasing CSC gene expression is
still not widely known. RAD6 is combined with several protein
ubiquitin ligases to regulate DNA repair and gene transcription. The overexpression of the RAD6 protein is due to chemotherapyinduced DNA damage. RAD6 high expression affected cancer cells
by cooperating with RAD18 to activate DNA repair through several
pathways such as the Fanconi Anemia pathway, Homologous Recombination, and the Translation Synthesis pathway [9,11].
In the pathway of enhancing stemness, RAD6 is associated
with RNF20/40 which increases stemness factors such as SOX-2,
and ALDHA1 through monoubiquitinating effects on histones that
cause epigenetic modifications and changes and further cause
gene transcription changes in chromatin structure. RAD6 also stabilizes and promotes core localization of the B-catenin (transcription factor) unclear mechanism. B-catenin is a protein involved in
the regulation and coordination of cell adhesion and gene transcription. Increased expression of stemness factors supports cancer cell survival in response to treatment with chemotherapeutic
agents [11].
Clark et al., (2018) investigated the role of RAD6 in chemoresistant ovarian cancer by inhibiting RAD6A and RAD6B in several
ovarian cancer. These cells showed decreased expression of CSC
markers, activation of DDR protein, and concomitant sensitivity to
carboplatin responses suggesting that RAD6 expression increases
after chemotherapy and causes chemo-resistance in cancer cells
through stimulating CSC protein expression and increasing DNA
repair activity [12]. The study by Somasagara et al., (2016) found
an association between chemo-resistance and increased RAD6 in
ovarian cancer cells through RAD6-mediated ubiquitin signaling,
which led to increased DDR and CSC protein expression. In addition, a higher RAD6 (⩾5,1) was also associated with a disease
recurrence rate of 70% [13]. Another study concluded that RAD6
is related to the severity of ovarian cancer, breast cancer, and melanoma. Rad6 levels were significantly increased in severe ovarian
cancer with platinum chemo-resistance [14].
RAD6 overexpression can increase stem cell characteristics,
aggressivity, metastasis, and relapse. The epigenetic influence of
RAD6 causes the ubiquitination of some histone variants which
then regulate genes related to DNA repair, cell resistance, and
chemo-resistance [14]. RAD6 is also closely related to RAD18, a
protein E3 ubiquitin ligase that regulates the DNA repair pathway
in Fanconi anemia and the BRCA gene in breast cancer [13], RAD6
was involved in breast cancer chemo-resistance in which researchers inhibited RAD6 with a small molecule inhibitor and found
an increased sensitivity to cisplatin [15]. In bladder cancer, it was
also found that overexpression of enzymes from the UBE2 group,
one of which was RAD6, could affect the growth of bladder cancer
cells. An experiment was carried out by stopping the expression
of UBE2, then the cells would stop growing in the G2/M phase
and increase the apoptosis of these cancer cells [16].
RAD6 is known to be weakly expressed in normal breast tissue
and cells, and its overexpression is associated with breast cancer
progression. RAD6 overexpression in breast cancer induces transformation and resistance to doxorubicin and cisplatin. A study
found that melanoma, a skin cancer tissue, has a high expression
of RAD6 and Melan-A and B-catenin by RAD6/Melan-A dual positivity [17]. Another study used OV90 and SKOV3 cell cultures with
RT-PCR, and immunofluorescence staining after chemotherapy
found that chemo-resistance ovarian cancer has high expression
of RAD6 [13].
The epigenetic effect of RAD6 causes the ubiquitination of histone variants H2A, H2AX and H2B which then regulates genes related to DNA repair, cell resistance, and chemo-resistance. Several
epigenetic molecules such as histone methylase and demethylase
are known to cause the release of RAD6 against ubiquitinated
histone-containing genes [14]. RAD6 is also closely related to
RAD18, a protein E3 ubiquitin ligase that regulates the DNA repair
pathway in Fanconi anemia and the BRCA gene in breast cancer.
RAD6 can cause ovarian chemo-resistance by stimulating monoubiquitylation of FANCD2 and PCNA proteins that play an important
role in DNA repair and DNA Damage Tolerance (DDT) mechanisms
related to platinum-based chemotherapy. RAD6 inhibition test
with a Small Molecule Inhibitor (SMI) was found to decrease DNA
repair signals, decrease CSC markers, and increase the sensitivity
of ovarian cancer patients to chemotherapy. Another pharmacological test with RAD6-selective Small-Molecule Inhibitor (SMI)
was performed on breast cancer and colon cancer. As a result,
Therapy with Smi Can Increase the Sensitivity of Breast Cancer
(TNBC) to cisplatin. In colon cancer, SMI also increases sensitivity
to platinum-based chemotherapy [18]. Thus, RAD6 can be a target
for gene therapy to treat chemo-resistance of ovarian cancer [13]
RAD6 is also related to breast cancer [15], melanoma [17], and
pulmonary cancer [19].
Overall, our study found that there is overexpression of RAD6
in the chemo-resistance of ovarian cancer both in flow cytometry
and immunohistochemistry study. RAD6 has a significant role in
activating several DNA repair pathways and is substantial in chemo-resistance in the ovarian cancer [20]. RAD6 overexpression
is associated with mitotic abnormalities and tumor progression
[12]. We found that there was a significant increase in RAD6 levels (p<0,05) in chemo-resistance patients. However, better ROC
and AUC results were found in immunohistochemistry RAD6, with
good Accuracy (AUC 0.875), significant (p<0,05), the sensitivity of
90%, and specificity of 84%.
To our knowledge, our study is the first study examining RAD6
in ovarian cancer directly from the blood by flow cytometry study
and from the fresh ovarian cancer tissue by immunohistochemistry. However, even though we found strong evidence from
both studies that RAD6 has correlations with ovarian cancer chemo-resistance from both studies, we still need further investigations because RAD6 ovarian cancer phenotype maybe not be the
only big cause of the chemo-resistance. RAD6 is a potential gene
therapy target for ovarian cancer but more research is also required to prove this.
Conclusion
We conclude that there is a significant relationship between
increased levels of RAD6 expression (p<0,05) with ovarian cancer
chemo-resistance. Logistic regression results indicate that RAD6 is
significantly associated with ovarian cancer chemo-resistance and
can be used as a good predictor of ovarian cancer chemo-resistance whereas RAD6 immunohistochemistry is a better predictor.
Declarations
Ethics approval: Ethical approval was granted by the Health Research Ethics Committee of the Universitas Indonesia, Cipto Mangunkusumo Hospital No. KET-230/UN2.F1/ETIK/PPM.00.02/2021,
March 15th, 2021.
Consent to participate: Informed consent was obtained from
all participants included in the study.
Consent for publications: Not applicable. We have no individual person’s data in the manuscript. All authors have consented
to publication.
Availability of data and materials: The datasets used and/or
analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest: The authors declare that they have no
competing interests.
Funding: The funding of the research was from all the authors.
We have no support in the form of grants, equipment, drugs, etc.
Authors’ contributions: The all authors’ contributions are
equal.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6): 394-424.
- Brett M R, Brett M R, Jennifer B P, Thomas A S, Jennifer B P, et al. Epidemiology of ovarian cancer: A review. Cancer Biology & Medicine. 2017; 14(1): 9-32.
- Kessous R, Laskov I, Abitbol J, Bitharas J, Yasmeen A, et al. Clinical outcome of neoadjuvant chemotherapy for advanced ovarian cancer. Gynecol Oncol. 2017; 144(3): 474-9.
- Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363(10): 943-53.
- Du Bois A, Baert T, Vergote I. Role of Neoadjuvant Chemotherapy in Advanced Epithelial Ovarian Cancer. J Clin Oncol. 2019; 37(27): 2398-405.
- Alberto mendivil wf, chad livasy. Gynecological Cancer Management - Identification, Diagnosis & Treatment. USA: Wiley black well. 2010.
- Meng E, Long B, Sullivan P, McClellan S, Finan MA, et al. CD44+/CD24- ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis. 2012; 29(8): 939-48.
- Abad E, Graifer D, Lyakhovich A. DNA damage response and resistance of cancer stem cells. Cancer Lett. 2020; 474: 106-17.
- Somasagara RR, Tripathi K, Spencer SM, Clark DW, Barnett R, et al. Rad6 upregulation promotes stem cell-like characteristics and platinum resistance in ovarian cancer. Biochem Biophys Res Commun. 2016; 469(3): 449-55.
- Shen JD, Fu SZ, Ju LL, Wang YF, Dai F, et al. High expression of ubiquitin-conjugating enzyme E2A predicts poor prognosis in hepatocellular carcinoma. Oncology letters. 2018; 15(5): 7362-8.
- Clark DW, Mani C, Palle K. RAD6 promotes chemo-resistance in ovarian cancer. Mol Cell Oncol. 2018; 5(1): e1392403.
- Clark DW, Mani C, Palle K. RAD6 promotes chemo-resistance in ovarian cancer. Molecular & Cellular Oncology. 2018; 5(1): e1392403.
- Somasagara RR, Spencer SM, Tripathi K, Clark DW, Mani C, Madeira da Silva L, et al. RAD6 promotes DNA repair and stem cell signaling in ovarian cancer and is a promising therapeutic target to prevent and treat acquired chemo-resistance. Oncogene. 2017; 36(48): 6680-90.
- Omy TR, Jonnalagadda S, Reedy M, Palle K. RAD6-mediated epigenetic reprogramming contributes to therapy-induced chemo-resistance in ovarian cancer. AACR. 2021.
- Haynes B, Gajan A, Nangia-Makker P, Shekhar MP. RAD6B is a major mediator of triple negative breast cancer cisplatin resistance: Regulation of translesion synthesis/Fanconi anemia crosstalk and BRCA1 independence. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2020; 1866(1): 165561.
- Gong YQ, Peng D, Ning XH, Yang XY, Li XS, et al. UBE2T silencing suppresses proliferation and induces cell cycle arrest and apoptosis in bladder cancer cells. Oncology letters. 2016; 12(6): 4485-92.
- Rosner K, Adsule S, Haynes B, Kirou E, Kato I, et al. Rad6 is a Potential Early Marker of Melanoma Development. Transl Oncol. 2014.
- Sanders MA, Haynes B, Nangia-Makker P, Polin LA, Shekhar MP. Pharmacological targeting of RAD6 enzyme-mediated translesion synthesis overcomes resistance to platinum-based drugs. Journal of Biological Chemistry. 2017; 292(25): 10347-63.
- Sasaki H, Moriyama S, Nakashima Y, Yukiue H, Fukai I, et al. Decreased Hrad6B expression in lung cancer. Acta Oncologica. 2004; 43(6): 585-9.
- Spencer SM, Somasagara RR, Tripathi K, Bachaboina L, Scalici JM, et al. Rad6 inhibition sensitizes ovarian cancer cells to platinum drugs by attenuating activation of multiple DNA repair networks. Gynecologic Oncology. 2016; 141: 67-8.