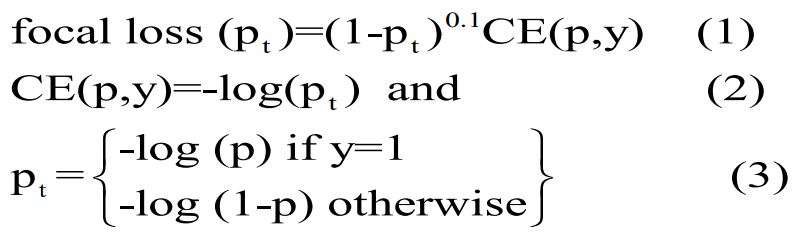Introduction
Endometrial Cancer (EC) is a common gynecological malignant
condition with a rising incidence worldwide [1]. High 5-year survival of EC patients relies on early diagnosis and treatment [2].
Atypical Endometrial Hyperplasia (AEH) is a precancerous condition of EC, and up to 40% of AEH would become EC without timely
hysterectomy [3]. Furthermore, approximately 30% of AEH would
develop into cancer within one year [4]. Considering the rapid
progress of EC and AEH lesions, the accurate detection of EC and
AEH is of utmost importance for early and effective diagnosis and
treatment.
Hysteroscopic-guided curettage has been widely considered to
be a useful tool to tailor treatment in patients with uterine malignancies. Hysteroscopy showed higher diagnosis performance
than that of Dilation and Curettage (D&C) alone [5]. A meta-analysis by Gkrozou et al. involving over 9,000 patients assessed the
accuracy of hysteroscopy in the diagnosis of polyps, submucosal
myomas, hyperplasia and endometrial cancer, demonstrating a
high diagnostic accuracy for endometrial cancer with a sensitivity
of 82.6% and a specificity of 99.7% [6].
However, misdiagnosis could underestimate the risk of uterine
conditions, leading to a treatment delay. A recent meta-analysis
on 1,106 patients, with a preoperative diagnosis of atypical endometrial hyperplasia, showed an underestimation of endometrial
cancer up to 32.7-45.3% following uterine curettage and hysteroscope guided biopsy [7]. Similarly, another systematic review
and meta-analysis evaluating D&C and hysteroscopy in diagnosing
cancer from women with postmenopausal bleeding, demonstrated that a high failure rate [11% (range 1-53%)] and infeasible endometrial samples [31% (range 7-76%)] would lead to a missing
diagnosis in average 7% (0-18%) of cases [8]. Considering the high
risk caused by missing diagnosis, a better diagnosis assist tool is
urgently needed for enhancing the accuracy of this evaluation.
Recently, deep learning has been widely applied in endoscopy,
especially for the detection of polyp, adenoma or gastrointestinal
cancer using colonoscopy, gastroscopy, hysteroscopy, and capsule endoscopy [9-11]. In a single center study on hysteroscopy,
a method was proposed for the classification of endometrial lesions and developed using 6,728 hysteroscopic images from 454
patients, and showed a 90.8% of accuracy, 83% of sensitivity and
96% of specificity for identifying lesions of benign or premalignant/malignant [10].
In this study, we performed a multicohort retrospective study
involving 1,446 cases from three tertiary hospitals for the development and validation of an Endometrial Cancer Computer-Aided Diagnosis (ECCADx) system based on deep learning for identifying AEH and EC from benign lesions.
Materials and methods
Study design and participants
This multicohort retrospective study was conducted in three
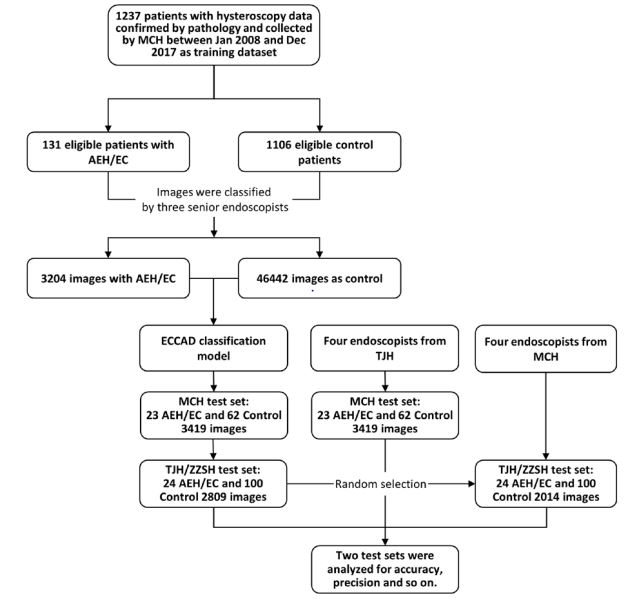
tertiary hospitals. A total of 1,446 cases with 55,874 images in png
format were enrolled consecutively. The numbers of cases and
images in training and test datasets were listed in Table 1. Images
were captured by one of three high resolution devices (Olympus
OTV-S190, Japan; Karl storz 26105FA or 26120BA, Germany). Pathological images of all lesions had been diagnosed by pathologists. All images have been confirmed by two experts WW.W. and
W.M. The control category (benign lesions) included cases with
endometrial polyps, submucosal uterine leiomyoma, endometrial
hyperplasia without atypia and normal uterine cavities (details
can be found in Table A1).
The training set was retrieved and collected from January 2008
to December 2017 at Maternal and Child Hospital of Hubei Province (MCH) by Olympus OTV-S190, Japan and Karl storz 26105FA
or 26120BA, Germany. The internal test dataset was made up of
images collected from January 2018 to June 2019 at MCH by the
same devices. The external test dataset contained data obtained
from January 2019 to December 2019 at Tongji Hospital of Huazhong University of science and technology (TJH) and the second
affiliated hospital of Zhengzhou University (ZZSH). AEH/EC categories included cases with endometrial atypia hyperplasia and
endometrial cancer. The external test datasets were mainly obtained by Olympus OTV-S190, Japan. The training and test datasets
have no case overlap.
We recruited four gynecological endoscopists from either
hospital of MCH and TJH to assess the counterpart’s test dataset
(TJH/ZZSH or MCH). The four endoscopists from either of the two
hospitals included two senior endoscopists with at least 15 years
of clinical experiences and two intermediate endoscopists with
more than 6 years of clinical experiences. This study is the first
attempt for multi-level evaluation of endometrial lesions. All eight
endoscopists evaluated all images of each patient as “Must be
benign”, “Most likely to be benign”, “May be benign”, “May be to
be malignant”, “Must be malignant”, according to their clinical experiences. Figure 1 illustrates the flowchart on the development
and estimate of ECCADx.
This study was approved by Medical Ethics Committee of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Maternal and Child Hospital of
Hubei Province and the Second Affiliated Hospital of Zhengzhou
University. To comply with the privacy policy, the training and analysis were conducted anonymously.
Training and test datasets
Detailed information of training and test datasets is illustrated
and listed in Figure 1 and Table 1. Additional two test datasets
include 3,419 images from 23 AEH/EC and 62 control cases diagnosed between January 2018 and June 2019 at MCH, and 2,809
images from 24 AEH/EC and 100 control cases diagnosed between
January 2019 to December 2019 at TJH/ZZSH, respectively. The
former test dataset serves as an internal test dataset, and the
latter one an external test dataset. Information of non-cancerous
disorders in the training and test datasets can be found in Table
A1. In the test datasets, all extracted images were put to use to
estimate the efficiency of ECCADx and endoscopists. The test datasets from MCH and TJH/ZZSH were evaluated by endoscopists
from TJH and MCH, respectively.
Model development
In this study, we proposed a convolutional neural network with
a backbone of ResNet-50 [12] for the analysis of hysteroscopic
images. ResNet-50 is a 50-layer convolutional neural network pretrained with over 100 million images in the ImageNet database [13]. Skip shortcuts used in ResNet50 [12] mimicking pyramidal
cells in cerebral cortex are employed to improve the performance
of convolutional neural networks. Image crops and resizing were
performed for all images in advance because images obtained by
different hysteroscopes have different image sizes and excessive
black background.
To overcome data unbalance caused by less cases of malignancies, i.e., AEH/EC, a focal loss in (1) multiplying the cross-entropy
function in (2)(3) with a modulating factor is used in the proposed
model to increase the sensitivity of misclassified AEH/EC observations [14]. Besides, an «over-sampling» technology was used
to compensate the impact of data imbalance in the training dataset [15]. We also employed image augmentation [16] by generating additional training data to prevent overfitting and improve
performance. Data augmentation was performed automatically
including image scaling, translation, rotation, and reflection. Furthermore, all training data was resized to 224*224 pixels to be
analyzed by ResNet50.
where p is the model’s estimated probability [14] for AEH/EC,
and y is ground truth (1: AEH/EC; 0: control).
For endoscopists, “Must be benign”, “Most likely to be benign”,
“May be benign”, “May be malignant”, “Most likely to be malignant”, “Must be malignant” were set with AEH/EC probabilities
of 0, 0.2, 0.4, 0.6, 0.8, and 1, respectively. These probabilities were
used to calculate Receiver Operating Characteristic (ROC) curves
and Area Under Curves of (AUC) of endoscopists.
The proposed method was developed with MATLAB R2020a
(The MathWorks, Inc. US), and Deep Learning Toolbox™ and Parallel Computing Toolbox™. We «freeze» the initial 10 layers in the
network by setting the learning rate to zero to prevent overfitting
and also speed up network training. A stochastic gradient descent
optimizer was used with a learning rate of 0.01, a momentum of
0.9, a decay rate of 0.1 every 10 epoch, and training epochs of 30.
The hyperparameters were set by trials and errors.
Statistical analysis
The classification efficiency of ECCADx was evaluated using
ROC curves, AUC, accuracy, sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), F1 score, Kappa,
Brier, and related 95% confidence interval (CI). All these statistical analyses were performed by R (version 4.0.2) programing language (R Development Core Team). Report ROC package (version
3.5) was used to calculate AUC, accuracy, sensitivity, specificity,
PPV, NPV; irr package (version 0.84.1) to calculate Fleiss’ Kappa
and two-sided z-test; measures package (version 0.3) to calculate
F1 score and Brier score; pROC package (version 1.17.0.1) to compute AUC, and confirm whether there is significant difference in
the AUCs between ECCADx and endoscopists using DeLong’s test.
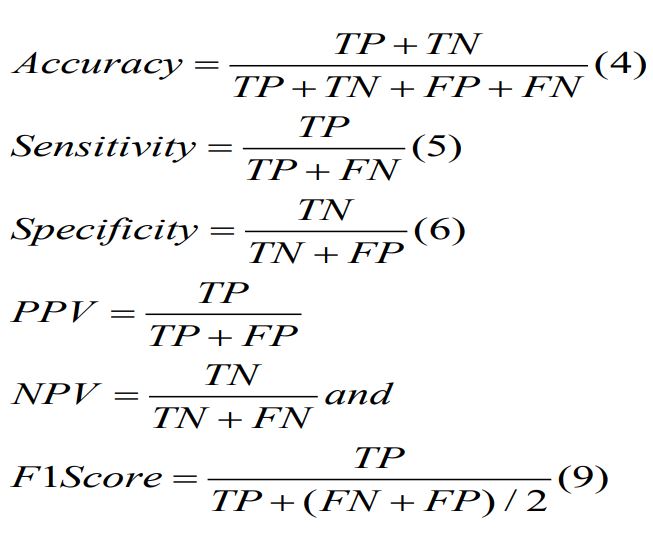
Accuracy, sensitivity, specificity, PPV, NPV are defined in the following equations.
Where TP, TN, FP, FN indicates true positive, true negative,
false positive, and false negative, respectively.
Finally, a predictive score of each lesion [16] was calculated
from predicted probabilities of images classified as malignancies,
i.e., AEH/EC. The predictive score is then used for the classification of AEH/EC and control.
Results
Performance of models on test datasets
ECCADx was trained and used to estimate the performance of
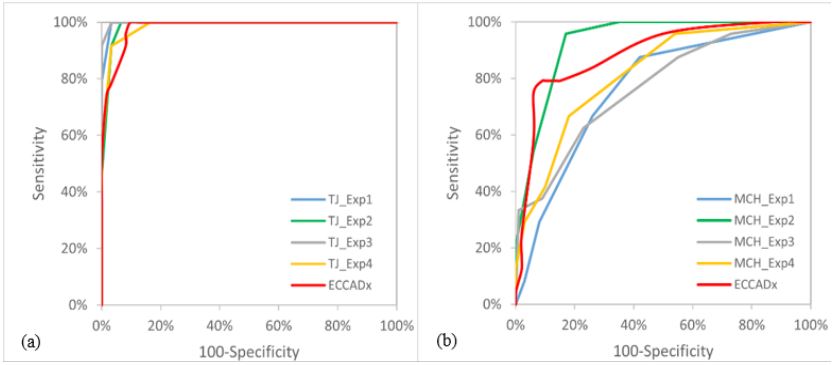
the proposed model on two test datasets. Figure 2 illustrates the
ROC curves of ECCADx and endoscopists in identifying AEH/EC.
As listed in Table 2, AUC value, accuracy, sensitivity, specificity,
and F1 of ECCADx was 0.965 (95%CI 0.931-1), 94.4% (95%CI 90.1-
98.8%), 92.8% (95% CI 85.7-100%), 92.5% (95% CI 86.7-98.3%)
and 0.939, respectively on the MCH test dataset. This indicated
a nearly perfect discriminative ability. No significant difference
was observed between the AUCs of ECCADx and endoscopists.
For TJH/ZZSH test dataset, the AUC, accuracy, sensitivity, specificity, and F1 were 0.881 (95% CI 0.789-0.972), 92.2% (95% CI 87.8-
96.7%), 75.2% (95% CI 59.5-90.8%), 95.2% (95% CI 91.5-99.0%),
and 0.826, respectively. No significant difference was observed
between the AUCs of ECCADx and the endoscopist (MCH-Exp2)
with the best performance. Other evaluation metrics such as PPV,
NPV, kappa coefficient and Brier were listed in Tables 2 and 3.
Six false negative cases of ECCADx included two cases with
polyp cystic degeneration, and one with myomatoid. There is no
abundant blood vessel distribution among them. The other two
false negative cases had typical lesions but poor image quality due
to necrotic tissues attached to the surfaces of lesions. The lesion
was missed in the images of the final case.
Performance of deep learning versus endoscopists
For MCH test dataset, we compared ECCADx with the TJH senior endoscopist with the largest AUC value in Table 2, AUC of
0.965 (95% CI 0.931-1) vs 0.974 (95% CI 0.947-1), accuracy of
94.4% (95%CI 90.1-98.8%) vs 95.6% (95%CI 91.8-99.4%), and F1
metric of 0.939 vs 0.958 as listed in Table 2. For TJH/ZZSH test dataset, ECCADx and one senior endoscopist from MCH reached an
AUC of 0.894 (95% CI 0.807-0.981) vs 0.881 (95% CI 0.789-0.972),
accuracy of 84.4% (95% CI 78.2-90.6%) vs 92.2% (95% CI 87.8-
96.7%) and F1 metric of 0.719 vs 0.826 as listed in Table 3. Other
evaluation metrics such as sensitivity, specificity, negative predictive value, and Kappa coefficient for ECCADx and endoscopists
were also listed in Tables 2 and 3. The interrater agreement rate
for the four experienced endoscopists from TJH was 62.4% (Fleiss’ Kappa 0.58; two-sided z-test, p<0.001) in MCH test dataset and
for four experienced endoscopists from MCH was 37.1% (Fless’s
Kappa 0.322; two sided z-test, p<0.001) in TJH/ZZSH test dataset.
Grad-CAM algorithm [17] was used to confirm important regions for predicting AEH/EC by ECCADx. These regions highlighted in Figure 3 may contain important morphological and vascular
features such as a gross distortion of endometrial cavity, focal necrosis, friable consistency, and atypical vessels related to different
pathological patterns of AEH and EC [18]. These features may play
a crucial role in ECCADx for recognizing AEH and EC.
Table A1: Information of non-cancerous disorders.
|
Training dataset |
MCH test dataset |
TJH/ZZSH test datase |
| P |
260 |
21 |
53 |
| NE |
499 |
41 |
36 |
| UL |
194 |
- |
9 |
| EH |
153 |
- |
2 |
P: Polyp; NE: Normal Endometrium; UL: Uterine Leiomyomata; EH:
Endometrial Hyperplasia.
Table 1: Baseline characteristics of training and test datasets.
|
MCH training dataset
|
MCH test dataset
|
TJH/ZZSH test dataset
|
|
AEH/EC1 |
Control |
AEH/EC |
Control |
AEH/EC |
Control |
| Cases |
131 |
1,106 |
23 |
62 |
24 |
100 |
| Images |
3,204 |
46,442 |
698 |
2,721 |
760 |
2,049 |
1AEH/EC: endometrial atypia hyperplasia and endometrial cancer
Table 2: Per patient diagnostic performance of endoscopists versus ECCADx in the MCH test dataset.
|
Gynecological endoscopist |
ECCADx |
| TJ-Exp1 |
TJ-Exp2 |
TJ-Exp3 |
TJ-Exp4 |
| AUC (95% CI) |
0.965(0.931-1) |
0.951(0.902-1) |
0.974(0.947-1) |
0.911(0.828-0.995) |
0.965(0.931-1) |
| P value (Exp vs ECCADx)* |
0.41 |
0.62 |
0.25 |
0.48 |
- |
| Accuracy (95% CI) |
94.4% (90.1-98.8%) |
92.2% (87.0-97.5%) |
95.6% (91.8-99.4%) |
85.4% (78.3-92.6%) |
94.4% (90.1-98.8%) |
| Sensitivity |
92.8% (85.7-100%) |
92.8% (85.7-100.0%) |
92.8% (85.7-100.0%) |
92.8% (85.7-100.0%) |
92.8% (85.7-100%) |
| Specificity |
92.5% (86.7-98.3%) |
89.5% (82.5-96.5%) |
94.0% (89.0-99.1%) |
80.4% (71.0-89.8%) |
92.5% (86.7-98.3%) |
| PPV (95% CI) |
83.5% (71.0-96.0%) |
78.2% (64.4-92.1%) |
86.4% (75.0-97.8%) |
65.9% (50.8-80.9%) |
83.5% (71.0-96.0%) |
| NPV (95% CI) |
97.0% (93.9-100%) |
96.8% (93.7-100.0%) |
97.0% (94.0-100.0%) |
96.5% (93.0-100.0%) |
97.0% (93.9-100%) |
| F1 |
0.939 |
0.902 |
0.958 |
0.807 |
0.939 |
| Kappa |
0.914 |
0.861 |
0.942 |
0.715 |
0.914 |
| Brier |
0.058 |
0.069 |
0.027 |
0.112 |
0.108 |
Table 3: Per patient diagnostic performance of endoscopists versus ECCADx in the TJH/ZZSH test datasets.
|
Gynecological endoscopist |
ECCADx |
| MCH-Exp1 |
MCH-Exp2 |
MCH-Exp3 |
MCH-Exp4 |
| AUC (95% CI) |
0.728 (0.605-0.85) |
0.894 (0.807-0.981) |
0.698 (0.571-0.824) |
0.709 (0.584-0.834) |
0.881 (0.789-0.972) |
| P value (Exp vs ECCADx) |
0.03 |
0.66 |
0.008 |
0.04 |
- |
| Accuracy (95% CI) |
63.3% (55.0-71.6%) |
84.4% (78.2-90.6%) |
73.4% (65.8-81.1%) |
55.5% (46.9-64.1%) |
92.2% (87.8-96.7%) |
| Sensitivity |
82.30% (69.0-95.7%) |
89.60% (79.8-99.3%) |
60.8% (42.7-78.8%) |
89.6% (79.8-99.3%) |
75.2% (59.5-90.8%) |
| Specificity |
57.70% (48.2-67.2%) |
81.8% (74.5-89.1%) |
76.0% (67.8-84.2%) |
46.2% (36.6-55.7%) |
95.2% (91.5-99.0%) |
| PPV (95% CI) |
34.2% (22.9-45.6%) |
56.8% (42.2-71.5%) |
40.4% (25.6-55.3%) |
30.8% (20.8-40.8%) |
81.0% (66.7-95.3%) |
| NPV (95% CI) |
92.4% (86.5-98.3%) |
96.7% (93.6-99.8%) |
87.8% (81.3-94.4%) |
94.2% (88.9-99.6%) |
93.4% (89.0-97.9%) |
| F1 |
0.483 |
0.719 |
0.484 |
0.455 |
0.826 |
| Kappa |
0.281 |
0.629 |
0.323 |
0.227 |
0.787 |
| Brier |
0.166 |
0.116 |
0.181 |
0.125 |
0.098 |
Discussion
The results demonstrated that ECCADx has a sensitivity and
specificity nearly equivalent to experienced endoscopists in identifying AEH/EC patients of 2 test datasets from different medical
centers.
The advantage of our ECCADx lies in recognizing AEH/EC from
non-cancerous lesions including polyps, submucosal uterine leiomyoma, endometrial hyperplasia without atypia, and normal uterine cavity. Moreover, the proposed system maintains the stability of diagnostic capabilities in datasets from different medical
centers. The combination of ECCADx and hysteroscopy systems
could balance the diagnostic efficiency of endoscopists with diverse working experiences and speed up the diagnosis process.
Meanwhile, the proposed system may serve as a second observer
to enhance the ability of endoscopists to deal with patients at high
risk of AEH/EC and reduce the misdiagnosis and unnecessary biopsy due to the perceptual bias and visual fatigue by endoscopists.
The establishment of ECCADx was based on the dataset over
9 years from a single hospital. Restricted sample size, population
distribution, discrepancy devices and uneven image quality would
lead to model instability in the analysis of other datasets. To comfirm this shortcoming, geographical and temporal test datasets
from two other hospitals as external test data were used here to
verify the classification ability of this model. The validation result
reflects a true diagnostic ability of ECCADx in processing images
from different devices with diverse quality and subject distributions. As we have introduced before, the training and internal test
dataset were obtained by Olympus OTV-S190, Japan or Karl storz
26105FA or 26120BA, Germany, and the external data by Olympus
OTV-S190, Japan. This may explain why ECCADx has less performance in validation using external dataset that that using internal
data. Nevertheless, EXCADx demonstrated nearly equivalent performance to experienced endoscopists.
Hysteroscopic-guided curettage can accurately remove benign
lesions and therefore reduce the probability of endometrial injury.
However, this may bring a risk of missed diagnosis for precancerous/malignant lesions, which are recommended to be removed
by hysterectomy and Bilateral Salpingo-Oophorectomy (TH/BSO)
[19]. Therefore, an underestimated diagnosis could lead to treatment delay. A computer-aided diagnosis system such as ECCADx
can play an important role in helping endoscopists identifying various precancerous and malignant lesions from benign ones.
Machine learning has been widely applied in gastrointestinal
endoscopy system for the detection and classification of disorders [20,21]. However, only very few studies were conducted in
hysteroscopy using computer-aided diagnosis. Neofytou et al.
presented a computer-aided diagnosis system for the early detection of endometrial cancer [22]. The CADx system was validated
using 516 Regions of Interest (ROIs) extracted from 52 subjects.
In terms of ROI classification, the best results were achieved by
using Statistical Features (SFs) and Gray-Level Difference Statistics
(GLDS) features with an SVM classifier. For this combination, the
proposed CAD system achieved an 81% correct classification rate
[21]. Recently, Ma et al.’s team used VGGNet-16 model to classify
endometrial lesions, and got a sensitivity of 84.0%, 68.0%, 78.0%,
94.0%, and 80.0% as endometrial hyperplasia without atypia,
atypical hyperplasia, endometrial cancer, endometrial polyp, and
submucous myoma [10]. Compared with these two models, ECCADx demonstrated superior performance for identifying endometrial cancer in a larger number of cases from multiple medical
centers.
In general, according to the morphological and vascular patterns of AEH and EC, gynecological endoscopists could recognize
AEH/EC from benign images. However, lower inter-rater agreement among gynecological endoscopists were observed. Especially for the testing results by the TJH/ZZSH test dataset, the agreement rates were from 48.3% to 71.8%. It might attribute to the
lower ratio of AEH/EC patients in TJH/ZZSH dataset (24/124) than
that in MCH dataset (23/85). This caused difficulty in identifying
cases with malignancy tumors. In addition, the proposed model
was trained by data only from MCH, and demonstrated lower
performance in the analysis of the data from TJH/ZZSH possible
because of inter-hospital difference mentioned above.
The limitations of this study are as follows
(1) Binary class model. ECCADx can only identify AEH/EC and
non-cancerous disorders. The next step is to distinguish atypical
hyperplasia and various pathological types of endometrial cancer,
which can better guide the treatment strategies.
(2) Retrospective study. The application and evaluation of ECCADx should be taken out in a multicentral prospective study in
the future.
Conclusion
The proposed ECCADx demonstrated satisfying performance
in identifying AEH/EC lesions from cases in different medical centers. The effectiveness of ECCADx was comparable or even better
than those of experienced gynecological endoscopists. In the future, this model should be validated in a prospective randomized
study in multicenter for the evaluation of its clinical usefulness.
Declarations
Funding: This study and APC was funded by National Natural
Science Foundation of China (No. 81701420), and the Competitive Research Fund from The University of Aizu (P-12-2022). The
funding sources are not involved in the performance of the research nor in the preparation of this manuscript.
Institutional review board statement: The study was conducted in accordance with the Declaration of Helsinki, and approved
by Medical Ethics Committee of Tongji Hospital Affiliated to Tongji
Medical College of Huazhong University of Science and Technology (Approval No. TJ-IRB20190604; Date: June 10th, 2019), and
Medical Ethics Committee of Maternal and Child Hospital of Hubei
Province (Approval No. [2022] IEC (007); Date: Feb. 10th, 2022);
was recorded at Institutional Review Board of the second affiliated hospital of Zhengzhou University (Approval No. 2022336;
Date: May 31th, 2022).
Informed consent statement: Patient consent was waived because the study was performed complying with the privacy policy,
and the training and analysis were conducted anonymously.
Data availability statement: The data used in this study is unavailable due to the rules of ethical approvals.
Acknowledgments: In this section, you can acknowledge any
support given which is not covered by the author contribution or
funding sections. This may include administrative and technical
support, or donations in kind (e.g., materials used for experiments). We also thank 8 endoscopists for diagnosing cases in two
test datasets.
Conflicts of interest: The authors declare no conflict of interest.
References
- Chen X, Xiang YB, Long JR, Cai H, Cai Q, Cheng J, et al. Genetic polymorphisms in obesity-related genes and endometrial cancer risk. Cancer. 2012, 118, 3356-3364. DOI: 10.1002/cncr.26552
- Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018, 178, 1210-1222. DOI: 10.1001/jamainternmed.2018.2820
- Giannella L, Delli Carpini G, Sopracordevole F, Papiccio M, Serri M, Giorda G, et al. Atypical Endometrial Hyperplasia and Unexpected Cancers at Final Histology: A Study on Endometrial Sampling Methods and Risk Factors. Diagnostics. 2020, 10. DOI: 10.3390/diagnostics10070474
- Gucer F, Reich O, Tamussino K, Bader AA, Pieber D, Scholl W, et al. Concomitant endometrial hyperplasia in patients with endometrial carcinoma. Gynecologic Oncology. 1998, 69, 64-68. DOI: 10.1006/gyno.1997.4911
- Nagele F, O’Connor H, Baskett TF, Davies A, Mohammed H, Magos AL. Hysteroscopy in women with abnormal uterine bleeding on hormone replacement therapy: a comparison with postmenopausal bleeding. Fertility and sterility. 1996, 65, 1145-1150. DOI: 10.1016/s0015-0282(16)58329-0
- Gkrozou F, Dimakopoulos G, Vrekoussis T, Lavasidis L, Koutlas A, Navrozoglou I, et al. Hysteroscopy in women with abnormal uterine bleeding: a meta-analysis on four major endometrial pathologies. Archives of gynecology and obstetrics. 2015, 291, 1347-1354. DOI: 10.1007/s00404-014-3585-x
- Bourdel N, Chauvet P, Tognazza E, Pereira B, Botchorishvili R, Canis M. Sampling in Atypical Endometrial Hyperplasia: Which Method Results in the Lowest Underestimation of Endometrial Cancer? A Systematic Review and Meta-analysis. Journal of minimally invasive gynecology. 2016, 23, 692-701. DOI: 10.1016/j.jmig.2016.03.017
- van Hanegem N, Prins MM, Bongers MY, Opmeer BC, Sahota DS, Mol BW, et al. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. European journal of obstetrics, gynecology, and reproductive biology. 2016, 197, 147-155. DOI: 10.1016/j.ejogrb.2015.12.008
- Min JK, Kwak MS, Cha JM. Overview of Deep Learning in Gastrointestinal Endoscopy. Gut Liver. 2019, 13, 388-393. DOI: 10.5009/gnl18384
- Zhang Y, Wang Z, Zhang J, Wang C, Wang Y, Chen H, et al. Deep learning model for classifying endometrial lesions. Journal of translational medicine. 2021, 19, 10. DOI: 10.1186/s12967-020-02660-x
- Soffer S, Klang E, Shimon O, Nachmias N, Eliakim R, Ben-Horin S, et al. Deep learning for wireless capsule endoscopy: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2020, 92, 831-839. DOI: 10.1016/j.gie.2020.04.039
- He K, Zhang X, Ren S and Sun J. Deep Residual Learning for Image Recognition. 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), 2016, 770-778. DOI: 10.1109/CVPR.2016.90
- Deng J, Dong W, Socher R, Li L, Li K, and Li F. ImageNet: A largescale hierarchical image database. 2009 IEEE Conference on Computer Vision and Pattern Recognition 2009, 248-255. DOI: 10.1109/CVPR.2009.5206848
- Lin T, Goyal P, Girshick R, He K, and Dollar P. Focal Loss for Dense Object Detection. 2017 IEEE® International Conference on Computer Vision (ICCV). Venice 2017, 2999–3007. DOI: 10.1109/ICCV.2017.324
- Ando S and Huang CY. Deep Over-sampling Framework for Classifying Imbalanced Data. In: Ceci M. HJ, Todorovski L., Vens C., Džeroski S. (eds), editor. European Conference, ECML PKDD. Skopje, Macedonia: Springer; 2017.
- Zhou D, Tian F, Tian X, Sun L, Huang X, Zhao F, et al. Diagnostic evaluation of a deep learning model for optical diagnosis of colorectal cancer. Nature communications. 2020, 11, 2961. DOI: 10.1038/s41467-020-16777-6
- Selvaraju RR, M. Cogswell, A. Das, R. Vedantam, D. Parikh, and D. Batra. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. Proc. IEEE International Conference on Computer Vision (ICCV), 2017, 618–626. DOI: 10.1007/s11263-019-01228-7.
- Garuti G, Mirra M, Luerti M. Hysteroscopic view in atypical endometrial hyperplasias: a correlation with pathologic findings on hysterectomy specimens. J Minim Invasive Gynecol 2006, 13, 325–330. 10.1016/j.jmig.2006.03.010.
- Magrina JF, Mutone NF, Weaver AL, Magtibay PM, Fowler RS, Cornella JL. Laparoscopic lymphadenectomy and vaginal or laparoscopic hysterectomy with bilateral salpingo-oophorectomy for endometrial cancer: morbidity and survival. American journal of obstetrics and gynecology. 1999, 181, 376-381. DOI: 10.1016/S0002-9378(99)70565-X
- Yahagi N. Is artificial intelligence ready to replace expert endoscopists? Endoscopy. 2021, 53, 478-479. DOI: 10.1055/a-1308-2121
- Gottlieb K, Requa J, Karnes W, Chandra Gudivada R, Shen J, Rael E, et al. Central Reading of Ulcerative Colitis Clinical Trial Videos Using Neural Networks. Gastroenterology. 2021, 160, 710-719. DOI: 10.1053/j.gastro.2020.10.024
- Neofytou MS, Tanos V, Constantinou I, Kyriacou EC, Pattichis MS, Pattichis CS. Computer-aided diagnosis in hysteroscopic imaging. IEEE Journal of Biomedical and Health Informatics. 2015, 19, 1129-1136. DOI: 10.1109/JBHI.2014.2332760.