Introduction
Breast Cancer (BC) has been growing in its incidence in the
past years. “In 2020, there were 2.3 million women diagnosed
with BC and 685,000 deaths globally” [1]. BC affects women in
a major proportion. There are three types of BC: the first type
expresses hormone receptors estrogen receptor (ER+) or Progesterone Receptor (PR+). The second type expresses Human Epidermal Receptor 2 (HER 2+) and the last one is the Triple Negative
Breast Cancer (TNBC) (ER-, PR-, HER2-) [2]. TNBC has six subtypes,
two Basal Like (BL1 and BL2), a Mesenchymal (M), Mesenchymal
Steam-Like (MSL), Immunomodulatory (IM) and Luminal Androgen Receptor (LAR) [3] (Figure 1).
The factors that influence BC activation are not clearly described, however the most common subtype is hormone receptor
positive with 60% of the documented cases, while HER2 positive
has an incidence of 20% and TNBC as only 10-20%, which has the
worst prognosis for the patient [2].
Patients with BC have a big number of treatments, depending
on the type of BC that they present. The pharmacotherapeutic approach can be based on the BC molecular characteristics with the
objective of improving the specificity and prognosis of the patient
[2].
Many treatments are approved by the US Food and Drug Administration (FDA), but for TNBC there are a few that are accepted,
and others are in different investigation stages, one of the reasons
is the unspecificity of chemotherapy. Scientists have researched
different ways to attack TNBC, and one way to treat this condition
is immunotherapy, in conjunction with chemotherapy [3].
TNBC treatment
Previously, the only treatment option for TNBC was surgery,
radiotherapy, and chemotherapy. Nevertheless, these therapeutic approaches did not recognize the difference between cancer
cells and normal cells, so they performed their effects on both
and caused unnecessary adverse effects in the patient associated
with their low specificity [4].
Traditional adjuvant or neoadjuvant chemotherapy for the
treatment of TNBC is based on the administration of anthracyclines with cyclophosphamide, and some regimens include taxanes [5]. These schemes achieve a favorable response in approximately 40% of cases, while the use of platinum reaches 50% but
has high toxicity.
In recent years, much research has been carried out with the
objective of increasing the selectivity and effectiveness of treatment, as well as reducing damage to the patient. Based on research and reports, immunotherapy was developed [6].
Immune response
Our body has a natural way to protect ourselves from tumor
cells, the responsible of this labor are T cells. They require two
signals to attack damaged cells, the first is to recognize an antigen,
then this is followed by CD28 costimulation.
Recent investigations suggest that some types of BC are immunogenically active and that some breast tumors have a sub-stantial lymphocytic infiltrate [7]. The first attempt to modify the
immune response in the research of a new treatment was blocking Cytotoxic T Lymphocyte-associated Antigen 4 (CTLA-4), this is
a checkpoint in the cytotoxic T-lymphocyte response. Other T-cell
checkpoints that modulate inhibitory signaling are programmed
cell Death Protein 1 (PD-1), mucin-domain containing 3 (TIM-3)
and Lymphocyte-Activation Gene-3 (LAG-3).
In recent years, many scientists have focused their investigations on the PD1/ Programmed Death Ligand 1 (PD-L1) pathway
[8].
PD-1 and PD-L1 in cancer immunotherapy
Immune evasion is an adaptive mechanism developed by tumor cells that allows them to escape the immune system and
consequently promote their survival and metastatic spreading [9],
which results in a poor prognosis for the patient.
Recent studies have showed that tumor cells can express high
levels of immune inhibitory signaling proteins as PD-L1, a transmembrane glycoprotein encoded by the Cd274 gen located on
chromosome 9 in humans [10].
PD-L1 interacts with PD-1 and generates a negative costimulation of T cell activation and reduce the cytokine expression such
as INF-ϒ, TNF-α and IL-2, which plays a key role in activation of
cellular immunity and subsequently, stimulation of antitumor
immune-response [11].
In the tumor microenvironment, the binding of PD-1/PD-L1
causes the activation of an evasion mechanism of the immune
system because it induces phosphorylation of tyrosine residues
in the cytoplasm, decreasing the induction of apoptosis of tumor
cells, inhibits secretion of granular enzymes, perforins and IFN-γ,
IL-2 and TNF-α [3].
Discovery of this negative regulation pathway of T cell activation provides a new opportunity for clinical application in diverse
advanced cancers, especially in patients with TNBC, with agonists
and antagonists of PD-1. The use of antibody-based PD-1 and PD-L1 inhibitors in clinical studies have shown durable tumor remissions in patients with TNBC [12].
Monotherapy or combination therapy as adjuvants or neoadjuvants represents a promising clinical alternative for the treatment of TNBC due to the remarkable clinical response in these patients. Nevertheless, these new strategies also are limited by
the social and economic accessibility of the patients, as well as
immune-related toxicity, innate and acquired drug resistance [12].
Immunotherapy is an ideal option for the treatment of TNBC,
because it allows selective inhibition of specific immune check-points, such as the documented expression of PD-L1 in tumor or
immune cells that allows it to evade the immune system [13].
Atezolizumab
Atezolizumab is a monoclonal antibody that blocks PD-L1 on
tumor cells or tumor infiltrating immune cells. Atezolizumab, Tecentriq, Genentech Inc., on March 8, 2019, the Food & Drug Administration (FDA) granted accelerated approval in combination
with paclitaxel protein-bound for adult patients with unresectable
locally advanced or metastatic TNBC whose tumors express PD-L1
> 1%. This approval was based on a phase 3 study, IMpassion130
(NCT02425891), multicenter, double-blind, placebo-controlled
study, with 902 participants randomized 1:1 in two-arm, for evaluated the efficacy, safety, and pharmacokinetics. The status of
the study is completed on August 31, 2021. Clinical benefit was
observed in patients who were treated with atezolizumab and
nab-paclitaxel demonstrated improved Overall Survival (OS) and
Progression-Free Survival (PFS) in both the Intention-To Treat (ITT)
population and the subgroup of PD-L1 positive population [14].
The data of the second prespecified interim OS analysis showed
that are consistent with the first interim OS analysis where although median OS in the ITT population was longer with Atezolizumab plus nab-paclitaxel than with placebo plus nab-paclitaxel,
the significance boundary was not crossed. However, the magnitude of OS benefit with atezolizumab in PD-L1 positive patients
remained clinically meaningful, with an increase of 7 months in
median OS with atezolizumab plus nab-paclitaxel treatment. The
updated PFS results in PD-L1 positive population showed an improvement of 2.2 months with atezolizumab plus nab-paclitaxel
compared with placebo group [15].
IMpassion031 (NCT03197935), is a global phase 3, double
blind, placebo-controlled study, with 333 participants randomized 1:1 in two arms, for evaluated the efficacy and safety of
atezolizumab or placebo in combination with neoadjuvant chemotherapy with nab-paclitaxel followed by doxorubicin plus cyclophosphamide for the treatment of early-stage TNBC. Atezolizumab with chemotherapy demonstrated improved pathological
complete response (pCR) in the ITT population in patients with
early TNBC including patients with PD-L1 negative [16].
IMpassion131 (NCT03125902), multicenter, phase 3, double
blind, placebo-controlled study, with 651 participants randomized
2:1 to receive atezolizumab or placebo plus paclitaxel in patients
with previously untreated inoperable locally advanced or metastatic TNBC. This study was designed to evaluate the efficacy and
safety of atezolizumab in combination with paclitaxel compared
with placebo plus paclitaxel. The data of the primary PFS analysis
showed that adding atezolizumab to paclitaxel did not show statistically significant improvement in investigator-assessed PFS in
the PD-L1 positive population. Final OS results was 22.1 months in
the atezolizumab plus paclitaxel group versus 28.3 months in the
placebo plus paclitaxel group [17]. Do to treatment with atezolizumab combined with paclitaxel did not significantly reduce the
risk of cancer progression and death, on September 8, 2020, the FDA alerted to health care professionals should not replace paclitaxel protein-bound with paclitaxel in clinical practice when use in
combination with atezolizumab [18].
Pembrolizumab
Pembrolizumab, Keytruda manufactured by Merck Sharp &
Dome Corp., is a monoclonal antibody that binds to the PD-1 receptor found on T cells and blocks its interaction with PD-L1 and
PD-L2. On November 13, 2020, the FDA granted accelerated approval to pembrolizumab in patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 combined positive score (CPS) > 10 [19]. This approval was based on
KEYNOTE-355 (NCT02819518) a phase 3, double-blind, multicenter, placebo-controlled study, with 847 participants, in part 2,
randomized 2:1 in two-arm, for evaluated the efficacy and safety
of pembrolizumab or placebo plus chemotherapy (nab-paclitaxel;
paclitaxel; or gemcitabine plus carboplatin). The data showed
that pembrolizumab plus chemotherapy clinically meaningful improvement in OS and PFS, in participants with CPS > 10 [20].
On July 26, 2021, FDA approved pembrolizumab for high-risk,
early-stage TNBC in combination with chemotherapy (carboplatin and paclitaxel followed by doxorubicin or epirubicin and cyclophosphamide) as neoadjuvant treatment, and then continued
as a single agent as adjuvant treatment after surgery [21]. This
approval was based on KEYNOTE-522 (NCT03036488) a phase 3,
double-blind, multicenter, placebo-controlled study, with 1174
participants randomized 2:1 in two arms. In the first interim analysis, in first 602 participants randomly, neo-adjuvant pembrolizumab in combination with chemotherapy followed by adjuvant
pembrolizumab improved in pCR (64.8% versus 51.2%) compared
with the neoadjuvant placebo in combination with chemotherapy
followed by placebo after surgery [22].
Ongoing clinical trials
Because patients with TNBC have high relapse rate and upon
relapse the median overall survival is less than a year, treatment
for the TNBC is the most challenging in comparison with breast
cancer expressing hormone receptor or human epidermal receptor 2 [23]. Based on the available clinical evidence and the recent
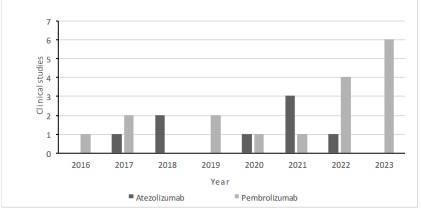
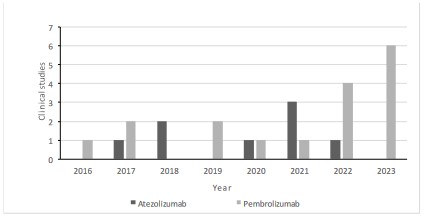
approvals for atezolizumab and pembrolizumab in TNBC, in Figure 2 we can observe the clinical trials that are proving atezolizumab or pembrolizumab as a treatment in conjunction with
other interventions, in the last eight years. On the other hand, there are many clinical trials evaluating PD-1/PD-L1 inhibitors to
continue the investigation of other Immune Checkpoint Inhibitor
(ICI) in combination with chemotherapy, vaccines, or even other
immunomodulatory drugs. Here, we summarize the ongoing and
recruiting clinical trials based on PD-1/PD-L1 inhibitors combined
with chemotherapy or with other therapeutic agents that are registered in ClinicalTrials.gov [24]. We excluded clinical trials with
not yet recruiting, completed, terminated, withdrawn, and unknown recruitment status. Table 1 includes some characteristics
of the clinical trials for anti-PD-1 and anti-PD-L1 antibodies.
Avelumab, toripalimab, nivolumab, sintilimab, spartalizumab,
tislelizumab, cemiplimab and durvalumab are antibody PD-1/PD-L1 inhibitors that we found with registered clinical trials. Each of
these antibodies is being evaluated in combination with chemotherapy-based regimen but also with other drugs that have different therapeutic targets. We found studies in different clinical
phases of development, most of them are in phase I and phase
II. The studies for avelumab, toripalimab and durvalumab in advanced clinical phases are described below.
The clinical trial phase III for avelumab as adjuvant or post-neoadjuvant treatment for high-risk TNBC (NCT02926196), is an
open label study with 474 patients who have completed treatment with curative intent including surgery followed by adjuvant
chemotherapy (stratum A) or including neoadjuvant chemotherapy followed by surgery further adjuvant chemotherapy (stratum
B). Treatment with avelumab will be given for 1 year. The primary
outcome measure is Disease Free Survival (DFS) and the secondary outcome measure is overall survival up to 5 years after randomization. The study started on June 2016 and the estimated
date to complete the study is June 2023.
The clinical trial phase III (NCT04085276), multicenter, double-blind study with 531 participants for evaluate toripalimab or placebo with nab-paclitaxel for first/second line treatment of metastatic or recurrent TNBC. The primary outcome measure is PFS
up to approximately 61 months from first patient in. The study
started on December 21, 2018, and the estimated date to complete the study is on December 31, 2024.
The clinical trial phase III (NCT05629585), is a multicenter,
open label, 3-arm study with 1075 participants for evaluate the
efficacy and safety of datopotamab deruxtecan with or without
durvalumab versus investigator´s choice of therapy (capecitabine,
pembrolizumab) in participants with stage I to III TNBC. The primary outcome measure is invasive Disease-Free Survival (iDFS); it
will be determined based on disease recurrence per investigator
assessment based on all available clinical assessments. The study
started on November 28, 2022, and the estimated date to complete the study is on March 27, 2030.
Undoubtedly, the findings and results of these studies will provide further insight into the benefit that PD-1/PD-L1 inhibitors can
have when combined with different drugs. On the other hand, it
is important to highlight that in the most ongoing clinical trials in
patients with TNBC, the therapies such as platinum, capecitabine,
gemcitabine, anthracycline, and taxane-based regimens combined with PD-1/PD-L1 inhibitors the activity in patients with
TNBC has been demonstrated.
Table 1: Baseline characteristics and demographics.
| |
|
Avelumab |
|
|
|
| Identifier |
Phase |
Treatment |
Condition |
Status |
Estimated study completion date |
| NCT05069935 |
I |
Avelumab and FT538 is an allogeneic natural killer cell immunotherapy |
TNBC |
Active, not recruiting |
August 5, 2025 |
| NCT04551885 |
I |
Avelumab and FT516 |
TNBC |
Active, not recruiting |
January 25, 2037 |
| NCT04360941 |
Ib |
Palbociclib Avelumab |
Metastatic androgenreceptor (AR)+TNBC |
Recruiting |
July 1, 2024 |
| NCT02630368 |
I/II |
Avelumab and JX-594 and cyclophospamide |
TNBC |
Recruiting |
November 2024 |
| NCT03475953 |
I/II |
Avelumab and regorafenib |
TNBC |
Recruiting |
December 31, 2025 |
| NCT05329532 |
I/II |
Modi-1/Modi-1v vaccines Avelumab |
TNBC |
Recruiting |
June 30, 2026 |
| NCT03971409 |
II |
Avelumab combined with binimetinib or anti-OX40 antibody PF-04518600 or utomilumab or doxorubicin or sacituzumab govitecan |
Metastatic TNBC |
Recruiting |
June 30, 2024 |
| NCT02926196 |
III |
Avelumab adyuvant or post-neoadjuvant |
High-risk TNBC |
Active |
June 2023 |
| |
|
Toripalimab |
|
|
| Identifier |
Phase |
Treatment |
Condition |
Status |
Estimated study completion date |
| NCT05103917 |
I/II |
Toripalimab with X4P-001 |
Locally advanced or metastatic TNBC |
Enrolling by invitation |
May 21, 2023 |
| NCT04418154 |
II |
Epirubicin and cyclophosphamide followed by albumin bound paclitaxel and toripalimab |
TNBC |
Active, not recruiting |
December 31, 2023 |
| NCT04085276 |
III |
Toripalimab with nab-paclitaxel |
Metastatic or recurrent TNBC |
Active, not recruiting |
December 31, 2024 |
| |
|
Sintilimab |
|
|
| Identifier |
Phase |
Treatment |
Condition |
Status |
Estimated study completion date |
| NCT05402722 |
II |
Sintilimab with eribulin |
Metastatic TNBC |
Recruiting |
June 30, 2023 |
| NCT05386524 |
II |
Sintilimab combined with bevacizumab biosimilar and pegylated liposomal doxorubicin |
Metastatic TNBC |
Recruiting |
March 15, 2025 |
| |
|
Spartalizumab |
|
|
| Identifier |
Phase |
Treatment |
Condition |
Status |
Estimated study completion date |
| NCT02936102 |
I |
PDR001 (spartalizumab) with FAZ053 (Anti-PD-L1) |
TNBC |
Active, not recruiting |
June 14, 2024 |
| NCT04802876 |
II |
Spartalizumab or tislelizumab |
Tumors with PD1-high mRNA expressing (TNBC) |
Recruiting |
March 31, 2027 |
| |
|
Cemiplimab |
|
|
| Identifier |
Phase |
Treatment |
Condition |
Status |
Estimated study completion date |
| NCT04243616 |
II |
Cemiplimab with chemotherapy (paclitaxel, carboplatin, doxorubicin, cyclophosphamide) |
TNBC |
Recruiting |
January 1, 2025 |
| NCT04916002 |
II |
Cemiplimab with vidutolimod |
TNBC |
Recruiting |
March 7, 2027 |
| |
|
Nivolumab |
|
|
| Identifier |
Phase |
Treatment |
Condition |
Status |
Estimated study completion date |
| NCT02637531 |
I |
Eganelisib with nivolumab |
TNBC |
Active, not recruiting |
December 2020 |
| NCT03667716 |
I |
In column for treatment the correct is: Nivolumab with COM701 (an inhibitor of poliovirus receptor related immunoglobulin domain containing (PVRIG)) |
TNBC |
Active, not recruiting |
December 2023 |
| NCT04925284 |
I |
Nivolumab with XB002 |
TNBC |
recruiting |
October 7, 2024 |
| NCT02393794 |
I/II |
Nivolumab combined with cisplatin and romidepsin (histone deacetylase inhibitor) |
Active, not recruiting |
Active, not recruiting |
July 2025 |
| NCT04331067 |
Ib/II |
Nivolumab and chemotherapy |
Early stage TNBC |
Active, not recruiting |
May 31, 2026 |
| NCT05329532 |
I/II |
Nivolumab or Pembrolizumab with Modi-1 vaccine (with potent anti- tumour activity) |
Advanced TNBC |
Recruiting |
June 30, 2026 |
| NCT04895709 |
I/II |
Nivolumab combined with BMS-986340 |
TNBC |
Recruiting |
September 15, 2026 |
| NCT05888831 |
I/II |
Nivolumab with BMS986449 |
Advanced, unresectable /metastatic TNBC |
Recruiting |
July 1, 2027 |
| NCT03487666 |
II |
Nivolumab with capecitabine |
High risk TNBC and residual disease after effective neoadjuvant chemothera py |
Active, not recruiting |
December 2022 |
| NCT03818685 |
II |
Nivolumab with Ipilimumab combined with radiotherapy |
TNBC with residual disease after neoadjuvant chemothera py |
Active, not recruiting |
March 1, 2024 |
| NCT03414684 |
II |
Nivolumab with carboplatin |
Metastatic TNBC |
Active, not recruiting |
June 30, 2025 |
| NCT02499367 |
II |
Induction treatment (radiotherapy, doxorubicin, cyclophosphamide, cisplatin) combined with nivolumab |
TNBC |
Active, not recruiting |
August 2025 |
| NCT03546686 |
II |
Ipilimumab with nivolumab pre-operative cryoablation. Post surgery only nivolumab |
Early stage resectable TNBC |
Recruiting |
June 2026 |
| NCT04159818 |
II |
Induction treatment with cisplatin or doxorubicin combined with nivolumab |
TNBC |
Recruiting |
December 15, 2026 |
| |
|
Durvalumab |
|
|
| Identifier |
Phase |
Treatment |
Condition |
Status |
Estimated study completion date |
| NCT03199040 |
I |
Durvalumab plus Neoantigen DNA vaccine |
TNBC |
Active, not recruiting |
August 23, 2023 |
| NCT03983954 |
I |
Durvalumab with naptumomab estafenatox |
TNBC |
Active, not recruiting |
August 30, 2023 |
| NCT02826434 |
I |
Durvalumab with PVX410 (vaccine) |
TNBC with human leukocyte antigen (HLA)-A2+ |
Active, not recruiting |
September 2023 |
| NCT04504669 |
I |
Durvalumab with
AZD8701 (FOXP3 antisense oligonucleotide) |
TNBC |
Active, not recruiting |
December 15, 2023 |
| NCT03739931 |
I |
Durvalumab with mRNA-2752 (a Lipid Nanoparticle Encapsulating mRNAs Encoding Human OX40L, IL-23, and IL-36γ) |
TNBC |
Recruiting |
April 1, 2025 |
| NCT03356860 |
I/II |
Durvalumab with chemotherapy
(paclitaxel, followed with epirubicin and cyclophosphamide) |
TNBC |
Active, not recruiting |
January 2023 |
| NCT03742102 |
I/II |
Durvalumab and paclitaxel combined with capivasertib or oleclumab.
Durvalumab combined with trastuzumab deruxtecan, or datopotamab deruxtecan |
Metastatic TNBC |
Recruiting |
August 15, 2024 |
| NCT03616886 |
I/II |
Durvalumab combined with carboplatin and paclitaxel with or without oleclumab (antiCD73) |
Advanced TNBC |
Active, not recruiting |
April 2025 |
| NCT04176848 |
II |
Durvalumab with CFI-400945 (blocking a specific protein called Polo-like Kinase 4 (PLK4) that is involved in cancer cell growth) |
Metastatic TNBC |
Active, not recruiting |
December 31, 2022 |
| NCT03606967 |
II |
Durvalumab with nab-paclitaxel, tremelimumab, and neoantigen vaccine |
Metastatic TNBC |
Recruiting |
December 31, 2024 |
| NCT05582538 |
II |
Durvalumab combined with ceralasertib and nab-paclitaxel |
Advanced TNBC |
Recruiting |
November 2025 |
| NCT03740893 |
II |
Durvalumab |
TNBC |
Recruiting |
December 2025 |
| NCT05215106 |
II |
Durvalumab |
Early small TNBC |
Recruiting |
June 2026 |
| NCT03801369 |
II |
Durvalumab with olaparib |
Metastatic TNBC |
Recruiting |
December 31, 2027 |
| NCT01042379 |
II |
Durvalumab combined with olaparib or datopotamab deruxtecan |
TNBC |
Recruiting |
December 2031 |
| NCT05629585 |
III |
Durvalumab with datopotamab deruxtecan |
TNBC |
Recruiting |
March 27, 2030 |
Conclusion
TNBC is a disease that represents a therapeutic challenge due
to its highly invasive nature, the non-expression of receptors that
leads to a low response to conventional therapy, and the expression of PD-L1 that contributes to evading the immune system.
Immunotherapy based on the use of immune point control
antibodies such as atezolizumab and pembrolizumab, allows to
regulate T cell regulatory mechanisms, blocking PD-1/PD-L1 has
represented an unprecedented advance in the treatment of TNBC
as shown in clinical studies that enhance the antitumor immune response, increase Overall Survival (OS) and Progression-Free Sur-
vival (PFS) in patients.
In addition, there are still many clinical trials in the research
phase, with the objective of discovering new combinations of
treatments, reducing adverse effects, and increasing specificity,
resulting in a more economical and safer intervention for the patient.
Conflict of interest: Authors declare no conflict of interest.
References
- World Health Organization. https://www.who.int/news-room/
factsheets/detail/breast-cancer. Breast Cancer. 2023.
- Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, et al.
Breast cancer: Biology, biomarkers, and treatments. International
Immunopharmacology. Elsevier B.V. 2020; 84.
- Popovic LS, Matovina-Brko G, Popovic M, Punie K, Cvetanovic A, et
al. Targeting triple-negative breast cancer: A clinical perspective.
Oncol Res. 2023; 31: 221-38.
- Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Vol. 15,
Human Vaccines and Immunotherapeutics. Taylor and Francis Inc.
2019; 1111-22.
- Di Cosimo S. Advancing immunotherapy for early-stage triple-negative breast cancer. The Lancet. Lancet Publishing Group. 2020;
396: 1046-8.
- Tarantino P, Corti C, Schmid P, Cortes J, Mittendorf EA, et al. Immunotherapy for early triple negative breast cancer: research agenda
for the next decade. Breast Cancer. Nature Research. 2022; 8.
- Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women with Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial.
JAMA Oncol. 2020; 6: 676-84.
- Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Human
Vaccines and Immunotherapeutics. Taylor and Francis Inc. 2019;
15: 1111-22.
- Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. PD-L1. J
Clin Pathol. 2018; 71: 189-94.
- Vargas-Rojas M, Jiménez-Álvarez L, Ramírez G, Torres-García D,
Barquera R, et al. PD-1 y sus ligandos como reguladores de la
respuesta inmune. Revista Instituto Nacional de Enfermedades
Respiratorias. 2008; 21: 272-9.
- Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-γ in tumor
progression and regression: A review. Biomark Res. 2020; 8: 49.
- Sun C, Mezzadra R, Schumacher TN. Regulation and Function of
the PD-L1 Checkpoint. Immunity. 2018; 48: 434-52.
- Wu M, Huang Q, Xie Y, Wu X, Ma H, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy
and PD-L1 regulation. Vol. 15, Journal of Hematology and Oncology. 2022.
- US Food & Drug Administration. U. S. Food & Drug Administration.
FDA approves atezolizumab for PD-L1 positive unresectable locally
advanced or metastatic triple-negative breast cancer. 2023.
- Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, et al.
Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast
cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
2020; 21: 44-59.
- Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracyclinebased chemotherapy versus placebo and
chemotherapy in patients with early-stage triple-negative breast
cancer (IMpassion031): A randomised, double-blind, phase 3 tri.
Lancet. 2020; 396: 1090-100.
- Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic
triple-negative breast cancer. Ann Oncol. 2021; 32: 994-1004.
- US Food & Drug Administration. U. S. Food & Drug Administration. FDA issues alert about efficacy and potential safety concerns
with atezolizumab in combination with paclitaxel for treatment of
breast cancer. 2023.
- US. Food & Drug Administration. U. S. Food & Drug Administration.
FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer.
2023.
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for
previously untreated locally recurrent inoperable or metastatic
triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;
396: 1817-28.
- US Food & Drug Administration. U.S. Food & Drug Administration.
FDA approves pembrolizumab for high-risk early-stage triple-negative breast cancer. 2023.
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med.
2020; 382: 810-21.
- Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, et al.
Breast cancer: Biology, biomarkers, and treatments. Int Immuno pharmacol. 2020; 84: 106535.
- NIH. National Library of Medicine. ClinicalTrials.gov. 2023.