Introduction
The term gastroblastoma was proposed and adopted in 2009
following the description by Miettinen of 3 cases of such a tumor
resected in 3 younger adult patients [1]. In his report, Miettinen
described these tumors as rare epitheliomesenchymal neoplasms
of the stomach that does not fit into recognized categories of biphasic tumors such as high-grade carcinosarcomas, sarcomatoid
carcinomas and synovial sarcomas [1]. Since then, 19 cases of gastroblastoma have been reported, most of them in young adults
and children [2,3]. The prognosis is uncertain, however, malignant
tumors have been described [4,5]. Herein we report the case of
a middle age woman with an incidentally found gastroblastoma.
Patient report: A female patient, 45-years-old, with gastritis
and gastroesophageal reflux symptoms, was submitted to perform
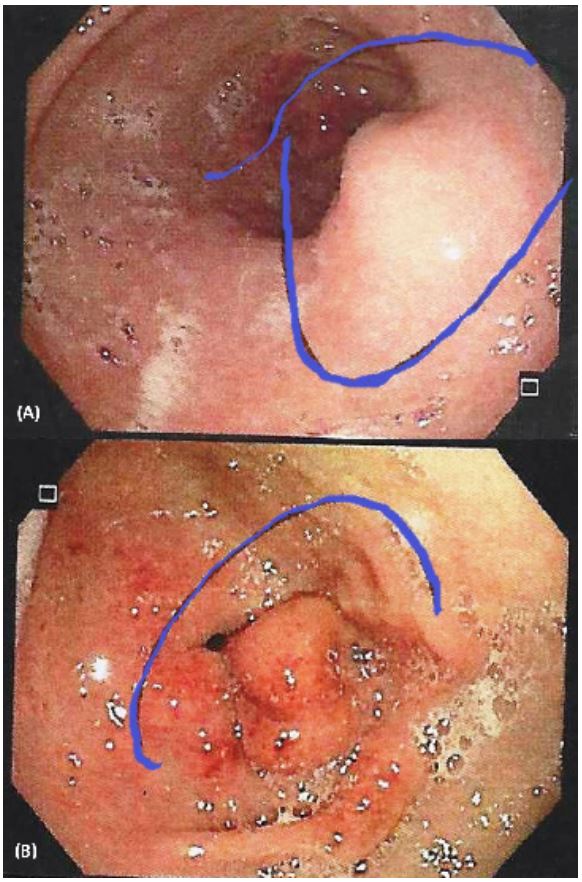
an upper endoscopy showing a 2 cm diameter submucosal lesion
in the antrum towards the posterior wall, eroded and covered
with fibrin (Figure 1). The biopsy reports mild active chronic gastritis with focal intestinal metaplasia and Helicobacter Pylori bacilli.
Evaluated at the surgery clinic, a computed tomography scan
(CT) of the abdomen was requested, reporting a hypervascular
endoluminal solid nodule on the anterior wall of the pylorus with
a diameter of 2 cm compatible with a gastrointestinal stromal tumor (GIST), and without any sign of dissemination.
Surgery was scheduled for laparoscopic antrectomy and Roux
Y reconstruction. The surgery was performed with endoscopic
control due to the endophytic growth of the tumor. The patient
was discharged on the 4th day.
The surgical biopsy reports diffuse sheets of uniform fusiform
and dominant spindle cells with round nuclei and the cytoplasm in
part eosinophilic, and clusters and cords of epithelioid cells. There
are no areas of necrosis. The mitotic index was 1 per high power field. Cellular immunoreactivity was positive for cytokeratins CK
AE1/AE3, CD56, CK7, Vimentin, and CD10. Cells were not reactive
to CK20, CDX2, S100, CD117, DOG1, Chromogranin, and Synaptophysin. With the diagnosis of neoplasm of undetermined origin,
new immunohistochemical studies on tumor cells were requested, reporting positive reactions with monoclonal antibodies to
Cytokeratins AE1/AE3, CD56, and CD10. Negative reactivity was
found for Chromogranin, Synaptophysin, SOX-10, and CD117. The
Ki 67 Cell proliferation index was 1% to 2%. With these studies,
the diagnosis of gastroblastoma was confirmed. Table 1, describes
some characteristics of our patient shared with other published
cases. Ten months after surgery, the patient is asymptomatic and
continues in follow-up.
Table 1: Summary of 20 cases.
| Author |
Year |
Age/
Gender |
Mitoses/
50 HPF |
Gastric
Location |
Size
(cm) |
Surgery |
Immunohistochemistry |
Follow-up (months)/
Outcome
|
| Miettinen et al. |
2009 |
19/M |
30 |
Greater
curvature |
5 |
Subtotal gastrectomy |
CD10, CK AE1/AE3, Keratin
18, Keratin 7
|
42/ANED |
| Miettinen et al. |
2009 |
27/F |
4 |
Greater
curvature |
6 |
Partial gastrectomy |
CD10, CK AE1/AE3, Keratin
18, Keratin 7
|
60/ANED |
| Miettinen et sal. |
2009 |
30/M |
1 |
Antrum |
15 |
Antrectomy |
CD10, CK AE1/AE3, Keratin
18, Keratin 7
|
168/ANED |
| Shin et al. |
2010 |
9/M |
1 |
Antrum |
9 |
Antrectomy |
CD10, CD56, CK AE1/AE3,
Vimentin
|
9/ANED |
| Wey et al. |
2012 |
28/M |
35 |
Antrum |
4 |
Antrectomy |
CD10, CD56, CK AE1/AE3, CK7,
Vimentin,
Chromogranin
A
|
3/ANED |
| Fernandes et al. |
2014 |
19/F |
5 |
Antrum |
10 |
Antrectomy |
CD10, CD56, CK AE1/AE3,
Vimentin
|
20/ANED |
| Ma et al. |
2014 |
12/M |
40 |
Antrum |
6 |
Antrectomy |
CD10, CD56, CK AE1/AE3, CK
CAM5.2,
Vimentin
|
8/ANED |
| Toumi et al. |
2017 |
29/F |
21 |
Cardia |
7 |
Partial gastrectomy |
CD10, C D99, Vimentin |
6/Deceased |
| Graham et al. |
2017 |
28/M |
3 |
Antrum |
4 |
Antrectomy |
CK AE1/AE3, CD56 |
Not reported/
Deceased
|
| Graham et al. |
2017 |
27/M |
1 |
Antrum |
9 |
Antrectomy |
CK AE1/AE3, SMA, GLI1 |
12/ANED |
| Graham et al. |
2017 |
9/M |
1 |
Antrum |
9 |
Antrectomy |
CK AE1/AE3, CD10, CD56,
Vimentin, GLI1
|
93/ANED |
| Graham et al. |
2017 |
56/F |
6 |
Antrum |
4 |
Biopsy only |
OSCAR, Vimentin, GLI1 |
Not reported/
Deceased
|
| Castri et al. |
2019 |
79/M |
1 |
Antrum |
9 |
Partial gastrectomy |
CD10, bcl2, CD56, CK
AE1/AE3, Vimentin
|
52/ANED |
| Centonze et al. |
2019 |
43/F |
2 |
Antrum |
5 |
Partial gastrectomy |
CD10, EMA, CK AE1/AE3, CK
CAM 5.2, CK7,
Vimentin,
GLI1
|
100/ANED |
| Pinto et al. |
2019 |
53/F |
2 |
Antrum |
2 |
Partial gastrectomy |
Vimentin, CD10, CD56, CK
AE1/AE3
|
18/ANED |
| Reardon et al. |
2020 |
22/F |
- |
Antrum |
7 |
Antrectomy |
CK AE1/AE3, CAM 5.2, GLI1
|
Not reported |
| Koo et al. |
2021 |
17/M |
1 |
Fundus |
6 |
Partial gastrectomy |
Vimentin, CD56, CD10, CK
AE1/AE3,
Synaptophysin.
|
23/ANED |
| Liu et al. |
2022 |
58/M |
5 |
Greater
curvature |
2 |
ESD |
Vimentin, CD10, bcl2, CD56,
CD100, EMA
|
Not reported |
| Sugimoto et al. |
2023 |
28/F |
1 |
Antrum |
7 |
Antrectomy |
CD10, Vimentin, CD56, GLI1,
PD-L1, CK AE1/
AE3,
HDCA2, CAM5.2, CK7
|
8/ANED |
| Present case |
2023 |
44/F |
1 |
Antrum |
2 |
Antrectomy |
CD10, CD56, CK7, Vimentin
|
10/ANED |
Discussion
Gastroblastoma is a sporadic epithelioid-mesenchymal biphasic gastric tumor composed of uniform spindle and epithelial
cells with an unclear etiopathogenesis, although it is believed that
a totipotent cell could be the origin [2,5-7]. Recently, Graham et
al, have confirmed that gastroblastoma is a distinct entity, and
demonstrated that represent translocation-associated tumors
characterized by the presence of a somatic, recurrent, oncogenic
MALAT1-GLI1 fusion gene, the presence of which causes over-expression of GLI1 protein and of several of its downstream targets
with key roles in tumorigenesis [8].
In most cases, this tumor has been reported in children or
young adult males with unspecific symptoms [1,3,5,7,8,11]. Only
some cases have been reported in older patients over 40-yearsold [9,10]. Our patient was an adult female with vague symptomatology. Clinically, the symptoms are dominated by epigastric
pain, impaired general condition, gastrointestinal bleeding, and
palpable mass if the tumor is large enough [1,3,5,12]. On upper
endoscopy, this tumor appears as a submucous mass, and the
overlying gastric mucosa may be normal or ulcerated, as with our
patient [7]. In other patients, this tumor is exophytic and not visible at endoscopy. In such cases, endosonography may be used
to perform biopsies [4,5,10,11]. In most patients, the tumor was
located at the antrum, as it was in our patient. Other locations
are the greater curvature and under the cardias [1,5,12]. Gastroblastoma had mesenchymal component and epithelial elements;
immunohistochemical markers such as Vimentin, CD10, CD56,
and cytokeratins are differently expressed in the two tumor components. A low-grade malignant potential is suspected based on
very few atypia, scarce mitosis, low ki-67 index, local growth pattern, and indolent clinical course [7]. Although, malignant behavior has been documented [4,5,6,8].
Radiologic studies such as and abdominal CT show the presence of a solid tumor with some cystic components arising from
the gastric wall with exophytic or endophytic growth and some
foci of calcification [5,6]. Tomographic characteristics are similar
to GIST, which is the tumor most often confused with gastroblastoma. In our case, radiologists also confounded the gastroblastoma with a GIST, which led us to the local resection that we performed. Other studies such as magnetic resonance might confirm
the tomographic findings, and better display the cystic components compared with CT [6,7].
Surgical resection with clear margins and lymphatic dissection
might the treatment of choice. Lymph node dissection should be
advocated because these tumors may spread via lymphatic vessels, and metastases to the liver and lymph nodes have been reported [5,6,8]. In one case, the outcome was the patient’s demise
due to metastatic dissemination [5]. However, gastroblastoma
seem to have a low and uncertain malignant potential despite
some reports on this tumor malignant behavior with ominous
outcomes [10]. The issue of whether the gastroblastoma should
be submitted to a second look surgery with lymphatic resection
after primary economic resection is perhaps the current controversial subject of its treatment. The laparoscopic approach or local resection might be suitable for tumors less than 5 cm away
from the gastroesophageal junction [1,2,9,10].
Macroscopically the gastroblastoma varies in size from a few
centimeters to 15 cm or more [1,5,8]. It can appear as ulcerated
endophytic masses, as polypoid tumors, intramural bulgings, or
exophytic excrescences [1,5]. Grossly these tumors are described
as multinodular or lobulated, and the cut surfaces varied from hemorrhagic to mottled surface [1,3,6]. The tumor originates in the
muscular gastric wall layer [9,10]. The histology of gastroblastoma
is biphasic, showing cell proliferation involving mesenchymal and
spindle to ovoid cells, which are dominant component [1,5,7,9].
The epithelial component is organized mainly in sheets, nests,
cords and tubules composed of glands, carpeted in places by the
cylindrical cells, other characteristics are the hyperchromatic nuclei and the slightly eosinophilic cytoplasm, and discrete nucleoli
[1,3,9]. In some cells, the nucleus and cytoplasm are condensed
and clearly bordered by separate cells [5,7]. The nuclei of glandular structures are dark and elongated [1,5]. The mesenchymal
cells are arranged in short fascicles or layers formed by oval cells
with scant cytoplasm with inconspicuous nucleoli and regular nuclei. No signs of structural differentiation such as fences or nuclear vacuolation are noted [3]. Both components showed blastemal immature appearance. These tumors had consistent clinicpathological features; occurrence in young adults, relatively large
tumor size, low-grade features with relatively low-mitotic activity
and low overall atypia, and lack of overt pleomorphism [1,9]; characteristics shared with our patient.
Immunohistochemical studies show vimentin and CD10 reactivity without any expression of muscle markers, CD34 or CD117
in the mesenchymal elements [1,8]. CD10 reactivity reflects fibroblastic phenotype [1]. Other commonly reactive markers in these
tumors are CK AE1/AE3, CD56, epithelial membrane antigen, and
CD117 in the epithelial components [3,7,8,10]. Neuroendocrine
differentiation is absent in gastroblastoma [1]. It has been suggested the use of the GLI1 immunochemical stain to diagnose limited
samples taken by cytologic aspiration by endosonography [11].
Several types of biphasic epitheliomesenchymal tumors are
known to occur in the stomach, the differential diagnosis includes
inflammatory myofibroblastic tumors, teratoma, gastrointestinal
stromal tumor, biphasic synovial sarcoma, and carcinosarcomas
[1,7]. Lymph node metastases have been reported in some cases,
as well as liver metastases and peritoneal carcinomatosis [4,5,8].
No standard therapy has been established for this tumor. The intra-peritoneal chemotherapy could reduce the loco-regional recurrence and peritoneal dissemination [5]. Some reported cases
received radiotherapy or chemotherapy with no response [1,4].
Gastroblastoma appears to have low malignant potential as recurrence after curative resection has seldom been reported [5].
The prognosis depends on several parameters including the size
of the tumor, the degree of parietal invasion, mitotic index, and
lymph node invasion; however, gastroblastoma seem to have a
low malignant potential. Probably long-term follow-up should be
advocated to avoid missing early and late recurrence.
Conclusion
Gastroblastoma is a distinct clinicopathological entity due to its
clinical, radiological, histopathological, and immunohistochemical
characteristics. Our case is the first reported case in Chile. To the
best of our knowledge, only 19 other cases have been reported
in the world literature. Despite the development of diagnostic,
morphological, immune-histochemical, and anatomopathological
techniques, diagnosis is often difficult. Gastroblastoma malignant
potential should be considered, and lymphatic dissection should
be performed at the surgery. In the case it was not performed,
long-term followup is important to avoid missing early or late recurrence.
Declarations
Conflict of interest: None.
Patient consent: Was obtained for publication of the
manuscript and all the images included.
Institutional Ethics Committee registration: 7C/004-23.
Funding: The authors declare that no funds, grants, or other
support were received during the preparation of this manuscript.
Authors contribution: All authors contributed to the study
conception and design. Material preparation, data collection and
analysis were performed by Marcelo A. Beltrán, and Constanza
Dictter. The first draft of the manuscript was written by Marcelo
A. Beltrán and all authors commented on previous versions of the
manuscript. All authors read and approved the final manuscript.
References
- Miettinen M, Dow N, Lasota J, Sobin LH. A distinctive epitheliomesenchymal biphasic tumor of the stomach in Young adults (“Gastroblastoma”). A series of 3 cases. Am J Surg Pathol. 2009; 33: 1370-1377.
- Sugimoto R, Uesugi N, Yamada N, Okasabe M, Baba S, Yanagawa N, et al. Gastroblastoma mimics the embryonic mesenchyme of the foregut: A case report. Diagnostic Pathol. 2023; 18: 24.
- Shin DH, Lee JH, Kang HJ, Choi KU, Kim JY, Park DY, et al. Novel epitheliomesenchymal biphasic stomach tumor (Gastroblastoma) in a 9year-old: Morphological, ultrastructural, and immunohistochemical findings. J Clin Pathol. 2010; 63: 270-274.
- Wey EA, Britton AJ, Sferra JJ, Kasunic T, Pepe LR, Appelman HD. Gastroblastoma in a 28-year-old man with nodal metastasis – Proof of malignant potential. Arch Pathol Lab Med. 2012; 136: 961-964.
- Toumi O, Ammar H, Korbi I, Ayed M, Gupta R, Nasr M, et al. Gastroblastoma, a biphasic neoplasm of the stomach: A case report. In J Surg Case Rep. 2017; 39: 72-76.
- Fernandes T, Silva R, Devesa V, Lopes JM, Carneiro F, Viamonte B. AIRP best cases in radiologic-pathologic correlation. Gastroblastoma: a rare biphasic gastric tumor. Radiographics. 2014; 34: 1929-1933.
- Ma Y, Zheng J, Zhu H, Dong K, Zheng S, Xiao X, et al. Gastroblastoma in a 12-year-old Chinese boy. Int J Clin Exp Pathol. 2014; 7: 3380-3384.
- Graham RP, Nair AA, Davila JI, Jin L, Jen J, Sukov WR, et al. Gastroblastoma harbors a recurrent somatic MALAT1-GLI1 fusion gene. Modern Pathol. 2017; 30: 1443-1452.
- Centonze G, Mangogna A, Salviato T, Belmonte B, Cattaneo L, Monica MA, et al. Gastroblastoma in adulthood – A rarity among rare cancers – A case report and review of the literature. Case Rep Pathol. 2019; AID 4084196: 1-6.
- Pinto DN, Diana-Gomes JV, Brito T, Leite M, Monteiro C, Matos C, et al. Gastroblastoma described in adult patient. Int J Case Rep Images. 2019; 10: 1-4.
- Reardon JD, Hatfield BS, Kraft AO, Smith SC. Gastroblastoma: Cytologic findings with resection and molecular correlation. Am J Clin Pathol. 2020; 154: 125-126.
- Liu Y, Wu H, Wu X, Feng Y, Jiang Q, Wang Q, et al. Gastroblastoma treated by endoscopic submucosal excavation with a novel PTCH1:: GLI1 Fusion: A rare case report and literature review. Curr Oncol. 2022; 29: 8862-873.