Introduction
Myasthenia Gravis (MG) is a neuromuscular junction disorder characterised by antibodies directed against postsynaptic
antigens (mainly the Acetylcholine Receptors (AChR)). One-half
of cortical thymoma patients develop Myasthenia Gravis (MG),
while 15% of MG patients have thymomas [1].
In thymomatous patients, whether with or without MG, elective thymectomy is mandatory with the dual aim of, firstly, ensuring oncological control of the neoplastic condition, and, secondly,
ameliorating or resolving myasthenic symptoms exhibited in MG
patients. While the removal of thymoma presents an oncological
“urgency», in the context of myasthenic patients, it is crucial to
undertake the operation solely when myasthenic symptoms are
effectively controlled by pharmacological therapy.
The management of MG symptoms relies on the utilization of
anticholinesterases (such as Pyridostigmine), immunosuppressive
and immunomodulatory therapies, including therapeutic plasmapheresis or high-dose human immunoglobulin. The adoption
of innovative and biological drugs remains in the experimental
phase.
Methods
In our investigation, a literature review was conducted on Pubmed using the Advanced tool, employing the keywords “thymoma” and “Steroid”, resulting in a total of 201 relevant works.
We specifically selected studies published in English from 1980 to
2024, with no restrictions on the number of included patients or
type of study. Ultimately, six studies met out inclusion criteria and
were selected (Table 1).
Table 1: Literature review.
| Author |
Year |
Country |
Kind of study |
N of patients |
Histology |
Kind of treatment |
Dimension reduction |
| Wrona et al. [7] |
2021 |
Poland |
Case report |
1 |
B1 |
low dose
prednisone (0.5/kg a day) |
75 to 30 mm |
| Kobayashi et al. [8] |
2005 |
Japan |
Prospective |
17 |
B1,
B2,
B3,
AB |
1 g metilprednis olone |
Reduction rate 2 to 85% |
| Qi et al. [9] |
2016 |
China |
Retrospective |
12 |
B2,
B3,
B1,
AB |
Pulse therapy |
Basic remission or marked improvement |
| Fujuwara et al. [10] |
2015 |
Japan |
Case report |
1 |
B1 |
Pulse therapy |
Improved reduction |
| Yoshida et al. [11] |
2012 |
Japan |
Case report |
1 |
B2 |
Prednisone |
Hyalinization |
| Kodama et al. [12] |
1997 |
Japan |
Case report |
1 |
|
Pulse therapy |
Complete remission |
Discussion
Thymic epithelial tumours represent the most common neoplastic lesions found in the anterior mediastinum, originating
from thymic epithelial cells. Over 90% of patients with thymoma
develop autoimmune diseases, with Myasthenia Gravis (MG)
being the most prevalent among them [2]. It has been reported
that approximately 30-50% of patients with thymoma develop
MG, whereas 10-20% of MG patients are found to have thymoma
[1,3]. In terms of therapeutic approaches, steroids and pyridostigmine have generally been used as mainstays for the control of
MG [4,5].
Kumagai and colleagues have described the use of immunosuppressive agents, such as azathioprine and methylprednisolone, in the treatment of thymomaassociated MG, demonstrating
notable improvement in clinical symptoms and reduction in tumour volumes [6].
Literature review
Among these, four were case reports, while one was a prospective study and another a retrospective one.
Wrona and colleagues [7] documented the impact of steroid
therapy on a patient affected by thymoma and autoimmune disorder. They observed a reduction in maximal tumour dimensions
after implementing steroid therapy (from 64x30x76 mm to 30x17
mm) and remission in infiltration on VCS, rendering the patient
eligible for surgery.
Kobayashi and colleagues [8] conducted a prospective study
to evaluate the efficacy of intravenous high-dose glucocorticoid
(steroid pulse) therapy in 17 previously untreated advanced thymoma patients. They found that preoperative steroid pulse therapy was most effective in type B1 thymoma, probably due to the
its specific effect on GR-rich CD4+8+ double-positive immature
lymphocytes.
A retrospective study from Qi and colleagues [9] analysed the
effect of steroid Pulse Therapy Plus Immunosuppressive Agent
for thymoma associated with MG in 12 patients. Their findings
suggested that steroid pulse therapy combined with immunosuppressive agents were effective and well-tolerated in treating both
metastatic thymoma and MG.
In the case described by Fujiwara and co-workers [10], the Authors report a case of a woman with an anterior mediastinal mass,
4.3 cm in diameter, with suspected invasion to the pericardium
and left brachiocephalic vein, along with multiple disseminations
to the mediastinal pleura and diaphragm, as well as pleural effusion. Following the first line of chemotherapy which included
cisplatin (30 mg/m2
, day 1), vincristine (1 mg/m2
, day 1), doxorubicin (40 mg/m2
, day 1), and etoposide (80 mg/m2
, days 1 to
3) administered over a 7-day cycle, the patient exhibited stable
disease. Consequently, as a second-line treatment, CAMP chemotherapy was initiated, comprising cisplatin (20 mg/m2
, days 1 to
4), doxorubicin (40 mg/m2
, day 1), and methylprednisolone (1,000
mg/body weight, days 1 to 4 and 500 mg/body weight, days 5 to
6). Additionally, due to respiratory failure attributed to MG, the
patient underwent two courses of methylprednisolone pulse therapy, followed by oral administration of prednisolone. After seven
months from the initial diagnosis, the patient became eligible for
surgery, as evidenced by a significant reduction in tumor size observed on CT scan.
Yoshida and colleagues [11] analysed the pathological findings
showing that prednisolone triggered a reduction in a B2 thymoma
by inducing apoptosis in both epithelial neoplastic cells and lymphocytic non neoplastic component of the thymoma.
In the case report by Kodama et al. [12], steroids were employed to treat the lung recurrence of a thymoma that had been
surgically resected 6 years prior. Remarkably, the patient exhibited a positive response both on lung nodules and on the newly
developed MG.
Results
To date, only a limited number of studies have investigated
the effect of steroids on tumour size, particularly in the context
of thymoma, which is relatively rare. Our case, described below, may contribute valuable insights to this field, as it demonstrates a
notable reduction in tumor size following steroid therapy, accompanied by significant improvement in MG symptoms.
Our experience
We present the case of a 53-year-old male patient admitted
to our ICU in June 2023 due to weakness in the lower limbs and
dysphagia, associated with respiratory failure necessitating endotracheal intubation and artificial ventilation. A clinical suspicion
of Myasthenia Gravis (MG) was raised. The assay for antibodies
against the Acetylcholine Receptor (AChR) yielded positive results, and electromyographic examination confirmed the diagnostic hypothesis of MG.
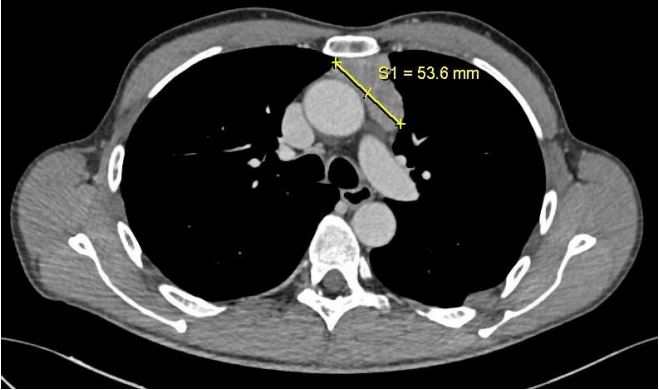
A whole-body CT scan was performed, revealing a solid tissue
with heterogeneous density in the anterior mediastinum, measuring approximately 53x41x70 mm, extending to the anonymous
vein without an evident cleavage plane (Figure 1).
Subsequently, the patient underwent standard therapy, receiving intravenous immunoglobulins, pyridostigmine 60 mg four
times daily, and prednisone 50 mg a day, resulting in partial clinical improvement.
Despite initial improvement, while undergoing a rehabilitation program, the patient experienced worsening in strength in
the upper limbs, along with the onset of dysphagia and rhinolalia.
In response, therapy was intensified, with prednisone increased
to 75 mg/day and pyridostigmine to 60 mg five times daily, but
no clinical benefit was observed. With the emergence of bulbar
symptoms, a cycle of plasmapheresis (and a cycle of intravenous
immunoglobulins (120 g) were initiated, resulting in modest clinical benefit. Due to persistent symptoms, the patient commenced
treatment with 2 infusions of Rituximab (1000 mg per infusion),
which yielded clear partial symptomatic relief.
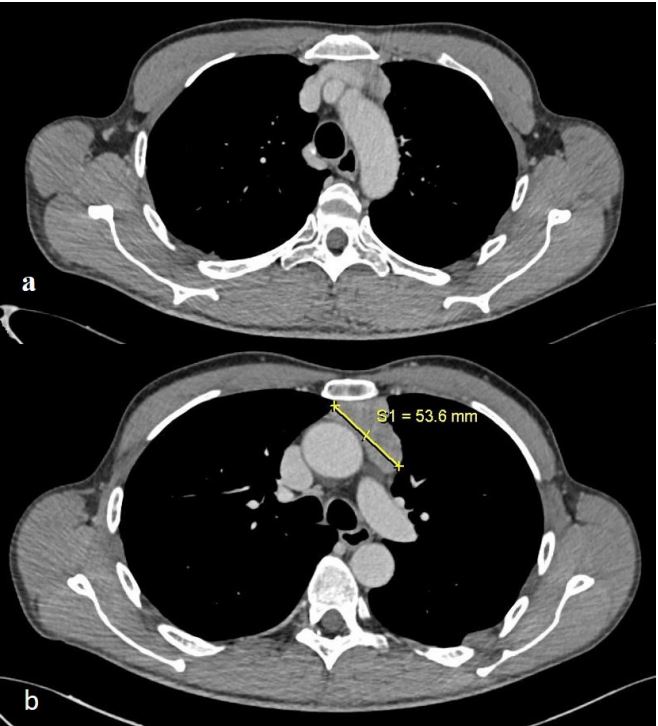
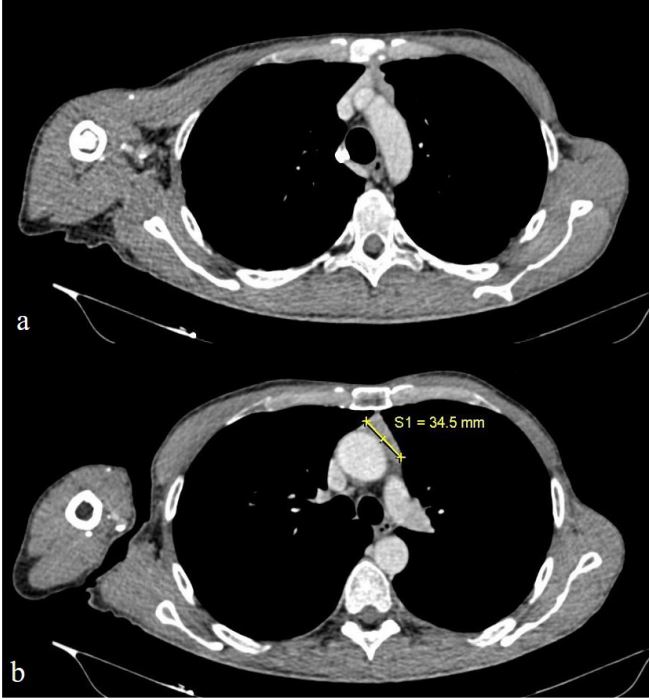
To monitor for potential neoplastic growth during the ongoing
therapy, the patient underwent a follow-up CT and PET CT scan in
October 2023. The scans revealed a reduction in the size of the
solid tissue, with no further contact with the anonymous vein
(measuring 34.5x16x70 mm compared to the previous 53x41x70
mm) (Figure 3). Additionally, a low-grade increase in metabolic
activity was noted in the tissue located in the anterior mediastinum (Figure 2).
In November 2023, due to inadequate symptomatic control of
MG, treatment with Ravulizumab was started, leading to clinical
benefit.
Considering the stabilization of the myasthenic condition, the
patient underwent re-evaluation for surgical intervention. Following a multidisciplinary assessment, the patient was deemed
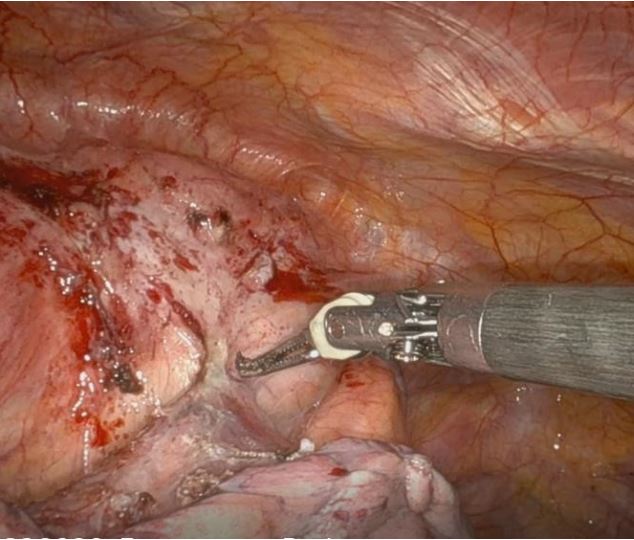
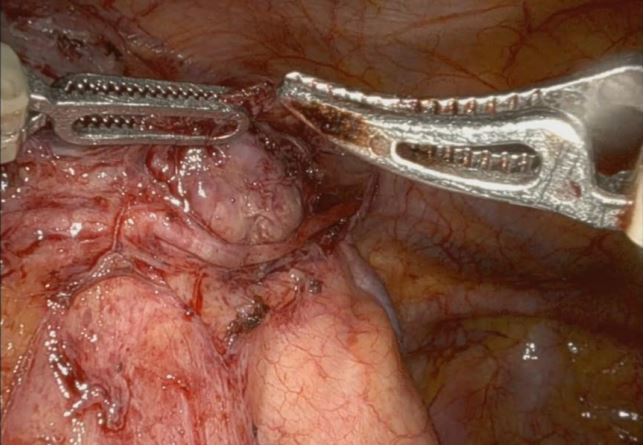
suitable for thymectomy. In January 2024, robot-assisted radical
thymectomy via a left approach was performed The procedure
involved radical thymectomy with resection of the thymoma,
thymus gland, and perithymic fat tissue while preserving the left
phrenic nerve, which was found to be encased by the thymoma
(Figure 4b) at the beginning of the surgical procedure. Moreover,
dissection of the Anonymous Vein was carried out successfully,
with no evidence of invasion by the thymic neoplasm.
In the immediate post-operative period, the patient underwent
multiparametric monitoring in ICU. On the first post-operative
day, he was transferred back to the ward without complications.
On the second post-operative day, the chest tube was removed,
and the patient was discharged home.
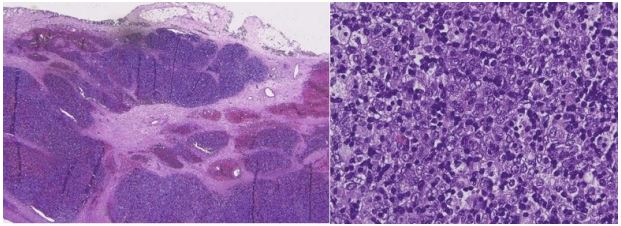
The definitive histological examination showed a thymoma,
classified as B2 type according to WHO, with a stage of pT1a Nx
(AJCC, 8th edition, stage IIb according to Masaoka-Koga) (Figure
5).
Conclusion
Our review serves as an interesting report highlighting the effect of corticosteroid on dimension of thymic epithelial tumour.
However, to further solidify and expand our understanding of
this phenomenon, studies involving larger cohorts would be invaluable. Such research endeavors would contribute to a more comprehensive consolidation of knowledge regarding the therapeutic
role of corticosteroids in managing thymic epithelial tumors.
References
- Filosso PL, Galassi C, Ruffini E, et al. Thymoma and the increased risk of developing extrathymic malignancies: A multicentre study. Eur J Cardiothorac Surg. 2013; 44: 219-24.
- Romi F: Thymoma in myasthenia gravis: From diagnosis to treatment. Autoimmune Dis. 2011; 2011: 474512.
- Marx A, Porubsky S, Belharazem D, et al. Thymoma related myasthenia gravis in humans and potential animal models. Exp Neurol. 2015; 270: 55-65.
- Sathasivam S. Steroids and immunosuppressant drugs in myasthenia gravis. Nat Clin Pract Neurol. 2008; 4: 317-27.
- Group MS. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008; 71: 394-99.
- Kumagai M, Kondou T, Handa M, et al. Treatment of invasive thymoma with myasthenia gravis: A case report responsive to azathioprine and methylprednisolone. Kyobu Geka. 1990; 43: 231-35.
- Wrona E, Dębska-Szmich S, Pastuszka M, Braun M, Czyżykowski R, et al. Remission of Thymoma on Steroid Therapy in a Patient with Atypical ThymomaAssociated Multiorgan Autoimmunity: A Case Report and Literature Review. Front Immunol. 2021; 12: 584703. doi:10.3389/fimmu.2021.584703.
- Kobayashi Y, Fujii Y, Yano M, Sasaki H, Yukiue H, et al. Preoperative steroid pulse therapy for invasive thymoma: clinical experience and mechanism of action. Cancer. 2006; 106(9): 1901-7. doi: 10.1002/cncr.21875.
- Qi G, Liu P, Dong H, Gu S, Yang H, et al. Metastatic Thymoma-Associated Myasthenia Gravis: Favorable Response to Steroid Pulse Therapy Plus Immunosuppressive Agent. Med Sci Monit. 2017; 23: 1217-1223. doi: 10.12659/msm.902442.
- Fujiwara A, Inoue M, Kusumoto H, Shintani Y, Maeda T, et al. Myasthenic crisis caused by preoperative chemotherapy with steroid for advanced thymoma. Ann Thorac Surg. 2015; 99(1): e11-3. doi: 10.1016/j.athoracsur.2014.10.022.
- Yoshida Y, Ueda R, Murakawa T, Ota S, Nakajima J. Thymoma hyalinized by steroid therapy in myasthenia gravis. Asian Cardiovasc Thorac Ann. 2012; 20(4): 479-81. doi: 10.1177/0218492312440187.
- Kodama K, Doi O, Higashiyama M, Yokouchi H, Yasuda T, et al. Dramatic response of postthymomectomy myasthenia gravis with multiple lung nodules to corticosteroids. Ann Thorac Surg. 1997; 64(2): 555-7. doi: 10.1016/S00034975(97)00555-9.