Introduction
In-situ follicular neoplasia (ISFN) is the monoclonal proliferation of B cells that present with similar immunophenotypic and
genetic features of follicular lymphoma but is confined to the
germinal centres of lymph nodes [1]. The characteristic genetic
alteration of this condition is t(14;18)(q32:q21), which is similar
to conventional follicular lymphoma [2]. The concept of in situ is
a Latin phrase meaning “on site” or “in position,” and when referring to cancer, it describes neoplastic cells that lack invasive
growth and are confined to the cell of origin [3]. In selected surgically removed, reactive lymph node samples, ISFN was identified
in approximately 2.8% of patients [4-6].
This condition was first described in 2002 [7]. It was detected
through immunohistochemical findings and molecular clonality analysis, as there is no disruption of surrounding tissue, and
the lymph node architecture is preserved [7]. In 2016, the World
Health Organization recognized this condition in their new classification guidelines, along with in situ mantle cell neoplasia [1]. It was considered a new condition because ISFN has a low rate of
progression, whereas partial involvement by follicular lymphoma
(PFL) is more likely to progress [2].
There are currently no established guidelines for the treatment
of this disease. Authors primarily suggest a “wait-and-see policy”
following the observance of ISFN when there is no evidence of
overt lymphoma [8]. One study treated patients with PFL with
local radiation therapy or rituximab and observed no remaining
evidence of disease following treatment [2]. This could potentially
be applied to ISFN, although more studies are needed to validate
the effectiveness of these treatment options.
In this report, ten cases of incidental in-situ follicular lymphoma from our cancer care institution (from 2005-2011) are
discussed. Morphological results, immunohistochemical findings,
computed tomography scans, and the significance of this diagnosis will be discussed.
Table 1: Patient data for ten patients with in-situ follicular neoplasia, the primary disease they presented with, and any findings that were
noted on a computed tomography (CT) scan.
| Patient |
Age |
Sex |
Presentation and primary disease |
Nodes taken |
CT findings |
| 1 |
58 |
F |
Intestinal adhesions (benign, post hysterectomy) |
Single enlarged node from small bowel mesentery |
No lymphadenopathy noted |
| 2 |
55 |
F |
Rectosigmoid resection (metastatic papillary serous
endometrial carcinoma) |
Mesenteric nodes, enlarged right common
iliac nodes |
Single right pelvic sidewall node with
enlargement from 10 mm to 15 mm |
| 3 |
68 |
F |
Radical hysterectomy (mixed serous/endometrioid
endometrial carcinoma) |
Bilateral pelvic lymph node dissection |
No lymphadenopathy noted |
| 4 |
63 |
F |
Radical hysterectomy, TAH, BSO (FIGO grade 2
endometrioid adenocarcinoma) |
Bilateral pelvic lymph node dissection |
No lymphadenopathy noted |
| 5 |
64 |
M |
Ruptured femoral artery aneurysm repair |
Enlarged inguinal lymph node |
No lymphadenopathy noted |
| 6 |
61 |
F |
Simple hysterectomy (TAH, BSO) with pelvic lymph
node dissection for FIGO 1 Endometrial Endometrioid
Adenocarcinoma |
Bilateral pelvic lymph node dissection |
No lymphadenopathy noted |
| 7 |
52 |
M |
Sentinel lymph node dissection for Malignant Melanoma |
Axillary lymph node dissection |
No lymphadenopathy noted |
| 8 |
72 |
M |
Radical Prostatectomy for Prostatic adenocarcinoma |
Bilateral pelvic lymph node dissection |
No lymphadenopathy noted |
| 9 |
68 |
M |
Radical Prostatectomy for Prostate adenocarcinoma |
Bilateral pelvic lymph node dissection |
No lymphadenopathy noted |
| 10 |
55 |
F |
Chronic Cholecystitis with cholelithiasis and lymph node
around cystic duct removed |
Mildly enlarged lymph node around cystic
duct |
No lymphadenopathy noted |
Case series
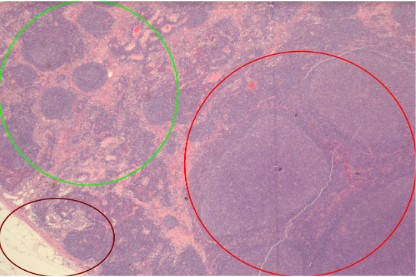
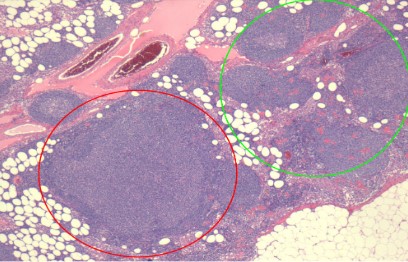
On microscopic examination, a large, reactive follicle was identified in the site of ISFN (Figure 1). As seen in Figure 2, there is a
nodular growth of neoplastic lymphoid cells that was identified.
The affected follicles have a similar shape to the unreactive follicles and nodal architecture is preserved.
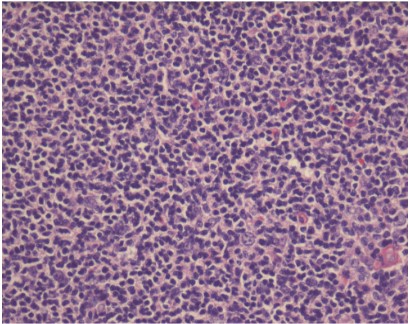
As seen in Figure 3, the neoplastic follicle shows no tingible
body macrophages, no polarization of cells with pale and dark
areas and monotonous small, cleaved cells.
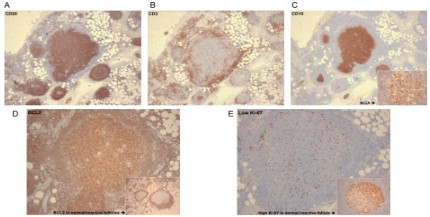
As seen in Figure 4A, the cells are positive for B cell marker,
CD20. Additionally, the reactive small T lymphocytes are highlighted by CD3 in Figure 4B. As seen in Figure 4C and the inset, the B
cell nodules express CD10 and BCL6, which are markers for germinal centre B cells. BCL2 is an antiapoptotic protein that is typically not expressed in reactive follicles. As seen in the inset of
Figure 4D, a normal follicle is negative for BCL2, whereas a follicle
with ISFN shows strong BCL2 positivity. The neoplastic follicle expresses low proliferation index compared to the reactive follicle
showing high proliferation index, as seen in Figure 4E.
Discussion
Our case series reports ten cases of incidental in-situ follicular
neoplasia. This precursor lesion was present in lymph nodes removed as part of the staging process of non-related diseases, as
outlined in Table 1. This rare disease was first described in 2002
as in-situ localization of follicular lymphoma (FL) in 23 patients and 18 with follow up [2]. In this series, five had synchronous FL
at another site, three developed FL after 3, 13 and 72 months respectively and two were with no known disease with 2-96 months
follow-up. This lesion is only found in 2% of reactive lymph nodes
examined [2]. It is defined as partial or total colonization of germinal centers by B cells with the BCL2 translocation; t(14;18).
There have been further discussions about whether a genetic hit
beyond t(14;18) is required for the in-situ component to become
an overt follicular lymphoma. Other case series have demonstrated similar results, a mixture of synchronous FL, subsequent FL,
and no progression. Some series have reported association with
subsequent non-FL lymphoma, e.g., splenic marginal zone, Hodgkin’s, DLBCL [9].
The diagnosis of ISFN is typically an incidental finding. In our
series, only one patient presented with lymphadenopathy on CT
scans, but three patients presented with enlarged lymph nodes
when extracted. The affected lymph nodes showed only partial
involvement by the precursor lesion with neoplastic cells confined
to the follicles admixed with reactive non-neoplastic follicles of similar appearance with preservation of normal nodal architecture.
All cases presented with similar immunohistochemical staining
patterns. The positive for staining for CD20, CD10 and BCL6 confirmed that these nodules were of follicular origin, and strong BCL2
of these follicles indicated the neoplastic nature of these follicles.
Additionally, a low Ki-67 score further signified the low-grade nature of this lesion compared to reactive follicles, where the proliferation index is high. In all our patients, no case progressed to
overt follicular lymphoma or transformed to diffuse large B cell
lymphoma (DLBCL). Follow-up staging of bone marrow biopsies
was negative for lymphoma.
IFSN may also indicate co-existing overt follicular lymphoma
in other locations. Additionally, the lymphoma may progress with
no evidence through CT scans. This highlights the importance of
a long-term follow-up and observation to better understand the
biological behaviour of the lymphoma. In our case series, none
of the cases had concomitant or synchronous lymphomas. The
lymph nodes were removed as part of the staging of unrelated
diseases (Table 1).
Conclusion
In conclusion, in-situ follicular neoplasia (ISFN) is an under-recognized lesion in the field of pathology. Previously, these patients
were labelled as lymphoma leading to unwarranted treatment. It
is now established that these are precursor lesions. The clinical significance and natural history of this entity are still under investigation. ISFN can be detected through subtle morphologic changes
noted in otherwise normal appearing follicles in a lymph node,
such as monotonous small cells confined to the follicle, loss of polarization and lack of tangible body macrophages. The morphology is further supported by immunohistochemical staining of BCL2,
CD10, BCL6 and low Ki-67 staining; the strong presence of these
proteins restricted to the germinal centres provides evidence for
ISFN. It is important that patients labelled with this diagnosis are
not overtreated with chemotherapy when diagnosed with ISFN.
Rather, patients should have continual hematological workups to
ensure that there is no follicular lymphoma elsewhere and be observed for disease progression without any active management.
References
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, et al. The
2016 revision of the World Health Organization classification of
lymphoid neoplasms. Blood. 2016; 127: 2375-2390.
- Jegalian AG, Eberle FC, Pack SD, Mirvis M, Raffeld M, et al. Follicular lymphoma in situ: clinical implications and comparisons with
partial involvement by follicular lymphoma. Blood. 2011; 118:
2976-2984.
- Oishi N, Montes-Moreno S, Feldman AL. In situ neoplasia in lymph
node pathology. Seminars in Diagnostic Pathology. 2018; 35: 76-83.
- Henopp T, Quintanilla-Martínez L, Fend F, Adam P. Prevalence
of follicular lymphoma in situ in consecutively analysed reactive
lymph nodes: Prevalence of follicular lymphoma in situ. Histopathology. 2011; 59: 139-142.
- Carvajal-Cuenca A, Sua LF, Silva NM, Pittaluga S, Royo C, et al. In
situ mantle cell lymphoma: clinical implications of an incidental
finding with indolent clinical behavior. Haematologica. 2012; 97:
270-278.
- Bermudez G, González de Villambrosía S, Martínez-López A, Batlle
A, Revert-Arce JB, et al. Incidental and Isolated Follicular Lymphoma In Situ and Mantle Cell Lymphoma In Situ Lack Clinical Significance. American Journal of Surgical Pathology. 2016; 40: 943-949.
- Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, et al.
In situ localization of follicular lymphoma: description and analysis
by laser capture microdissection. Blood. 2002; 99: 3376-3382.
- Carbone A, Santoro A. How I treat: diagnosing and managing “in
situ” lymphoma. Blood. 2011; 117: 3954-3960.
- Montes-Moreno S, Castro Y, Rodríguez-Pinilla SM, García JF, Mollejo M, et al. Intrafollicular neoplasia/in situ follicular lymphoma:
review of a series of 13 cases. Histopathology. 2010; 56: 658-662.